Efficient, effective healthcare with Timestrip®
Whether in a major teaching hospital, a walk-in clinic or a doctor’s surgery, any type of healthcare boils down to two broad objectives: improved patient outcomes and the best possible use of resources.
Although historically reconciling patient health and the harsh realities of budgetary constraints have at times led to difficult choices, the fact is that by integrating technology and innovation into decision making around resource allocation, both objectives can be achieved at the same time.
Indeed, thanks to its high tech / low cost Time and Temperature Indicator labels, Timestrip® is able to provide healthcare providers and the pharmaceutical sector with a broad range of practical, user-friendly solutions that:
✔ Ensure product quality
✔ Reduce product waste
✔ Ensure regulatory compliance
✔ Reduce time and temperature monitoring costs
But what does this look like in practice?
Improving quality control and efficiency

A bag of whole blood with a Timestrip® Blood Temp 10°C
Tenet Healthcare is US healthcare provider that faced the challenge of needing to effectively manage how it processed bags of whole blood. On the one hand, it faced the need to comply with local regulatory requirements related to the use of effective temperature monitoring for blood products; and on the other, it had to discard any bag of blood that had been exposed to a temperature above 10°C for more than 30 minutes.
The company needed to be absolutely certain that any unused bag of whole blood was 100% safe to be returned to storage for future use. It had also noted ongoing difficulties in using other blood temperature indicators. Potentially, the latter could lead to doubts about product safety, which in turn would require that bag of blood to be discarded, possibly unnecessarily.
Its use of Timestrip® Blood Temp 10 – 10°C Blood Irreversible Transit Temperature Indicator noted the following advantages:
✔ Improved quality of care
✔ Improved workflow efficiency
✔ Maintenance of quality control during transportation and storage
✔ Support in complying with regulatory guidelines
Cost-effective regulatory compliance

Marathon Distributors is a pharmaceutical and healthcare logistics services provider in Cyprus and is involved in the distribution of pharmaceutical products throughout the island. The main cold chain management issue it faced was around compliance with local and EU regulations, specifically the country’s Law on Human Pharmaceuticals 70(I) 2001 and the EU guidelines on Good Distribution & Manufacturing Practices. These mandated the company to ensure that the pharmaceuticals it delivered were not exposed to a temperature above 8°C for more than 8 hours during transportation.
Although other products helped achieve regulatory compliance, the company began using Timestrip® PLUS™ 8°C 8 Hrs (TP065) Irreversible Time and Temperature Indicators because they were much more competitively priced.
Overall the company reported:
✔ Timestrip Indicators were 50% cheaper
✔ Delivery costs were 50% lower
✔ Delivery times were faster
✔ Positive end-user feedback around ease of use
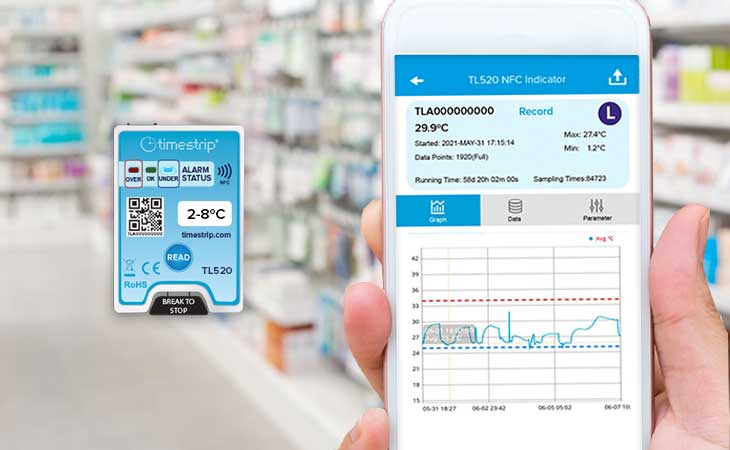
Easy temperature monitoring for vaccine transport
Cambridgeshire Community Service NHS Trust is a UK healthcare provider in south-east England. Part of its work is the delivery of school-age immunisations to schools in its area. This includes for instance flu nasal immunisations for primary schools and HPV vaccinations and Meningitis ACWY and TdIPV for secondary schools.
For the delivery of vaccines, electric Labcold Portable Vaccine Carriers and pharmaceutical-grade vaccine carriers are used. In order to maintain the efficacy of the various transported vaccines, two different temperature ranges need to be managed: 2°C to 8°C and -25°C to 25°C.
In order to ensure the delivery of viable, unspoiled vaccines, the Trust thus had a requirement for a dual, low-cost solution for use by its delivery teams.

To achieve this, two Timestrip products have been used in each vaccine carrier:
● a Timestrip® PLUS (TP 065) to indicate if the temperature went above 8°C
● a Timestrip® PLUS (TP 217) to indicate if the temperature exceeded 25°C, and if so, for how long
The feedback from the delivery teams has been extremely positive, specifically around:
✔ Easy to read visual data
✔ Ease of use
✔ Reliability
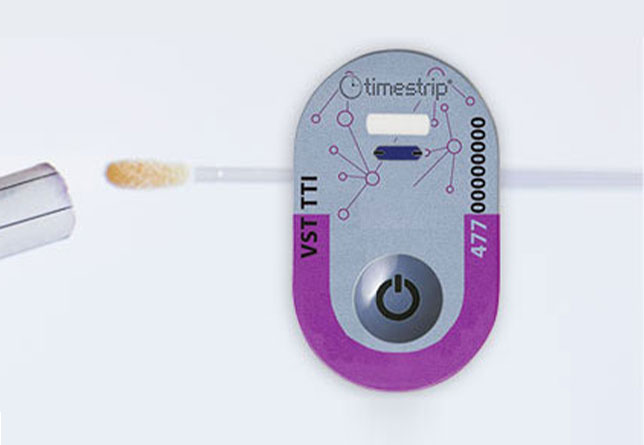
Reliable test kit time data
Clinical Innovations is a US maker of obstetrics devices that wanted to develop a non-invasive test kit for the diagnosis of any rupture of membranes (ROM). Although treatable, spontaneous ROM is a serious medical condition and a major cause of preterm births. Thus, an accurate diagnosis of ROM can be a key factor in determining timely and appropriate medical intervention. Similarly, a false diagnosis of ROM can lead to unnecessary hospitalisation, medication and even induced premature delivery.
Timestrip was able to provide Clinical Innovations with Time Indicators for its ROM Plus® self-contained test kit. These give clinicians the essential and immediate data they need to reduce the dangers of any premature ROM for expectant mothers and their babies.

The test kit uses Timestrip technology with an in-built 20-minute time monitoring indicator that generates the required data within 5-20 minutes, with 5,10 and 20 minutes clearly indicated on the Timestrip. Its key benefits are:
✔ Clear visual data
✔ Ease of use
✔ Improved health outcomes
Hygienic healthcare environment
Beyond pharmaceuticals and blood products, healthcare also involves various types of disposable items that need replacing regularly. Especially for the sick, hygiene in the clinical environment is an essential aspect of patients being able to make a full recovery. Timestrip indicators make excellent cleaning reminders for surfaces, filters and instruments.
Assuming the absence of any complications, catheters for instance need to be replaced every 30 days for Foley catheters and every 60-90 days for silicone catheters. Catheter bags however need to be replaced every 5-7 days. If these items are not replaced at appropriate intervals, opportunities are created for harmful pathogens to develop.

Unsurprisingly, this has also been shown to be the case for hospital privacy curtains. Dr Kevin Shek was the lead study author into the rate of contamination of hospital privacy curtains in a burns/plastic ward. In a report about the study findings in Nursing Times, he said:
“We know that privacy curtains pose a high risk for cross-contamination because they are frequently touched but infrequently changed. The high rate of contamination that we saw by the fourteenth day may represent an opportune time to intervene, either by cleaning or replacing the curtains."
Especially in busy hospital departments such as Accident & Emergency and ICU, hospital privacy curtains can quickly become contaminated with bacteria such as methicillin-resistant S. aureus (MRSA) and Clostridium difficile (C. diff). Thanks to Timestrip® Time Monitoring Indicator panels that change color after a set period of time, clinical staff are alerted when a privacy curtain needs to be changed. They help a hospital achieve:
✔ Best use of resources with a low-cost solution
✔ Compliance with guidelines around frequency of change
✔ Less time needed to monitor when that change needs to occur
✔ Hygienically clean hospital environment for patients