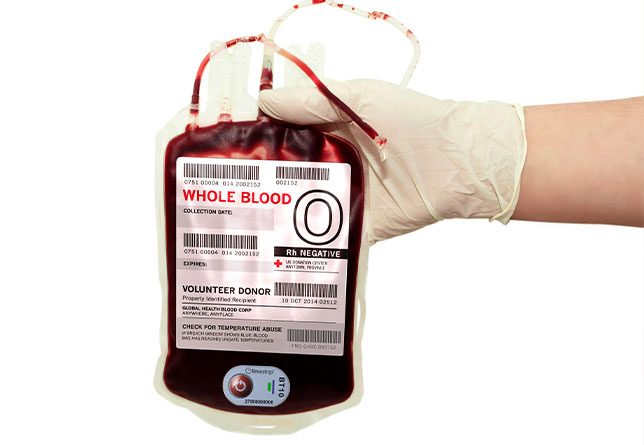
21st century blood temperature monitoring - Part 1
Interview with Reuben Isbitsky, founder of Timestrip UK Limited, which has just received FDA clearance for its 10°C Nonreversible Blood Transit Temperature Indicator.
What does FDA clearance of Timestrip 10°C mean for your company?
It’s major milestone. Product-wise, we've been deemed and certified as having a medical device that has an accuracy level of ± 0.5°C and is suitable for transporting whole blood. FDA clearance for Timestrip 10°C will allow us to market this product for this specific application.
And for our business, it marks a very important turning point. We started off as a time indicator company, which evolved into a temperature monitoring business, that is now a high-end medical device and healthcare business.
Moving forward, our business model will be to configure new products for specific and invariably speciality uses. We’re also going to be expanding our range of products for specific uses to enable the safe transportation of the different components of whole blood as well, namely platelets, plasma and frozen blood products. This will go a long way to maximizing the usability of all these components. For instance, even properly transported and stored whole blood only has a shelf life of 35 days, but plasma can be frozen and has many uses.
Lastly, for markets outside the US FDA clearance gives our product enormous credibility. Although FDA clearance cannot be enforced outside the US, in fact for many markets such as Saudi Arabia and India, it is used as an indicator of credibility and for tendering processes is even a prerequisite.







