Medicines exposed to the wrong conditions – such as the high temperatures now found in transporting vehicles - can be damaged and endanger health. This excellent piece from the New York Times explains the problem (ten minute read).
Don’t have ten minutes? Read this two minute version instead:
Top pharmaceutical temperature shipment takeaways
- Doctors and pharmacists say the scorching temperatures enveloping the country could be endangering people’s health in an unexpected way: by overheating their medications.
- Millions of Americans now receive their prescription medications through mail-order shipments, and this method is increasingly required by health insurance plans.
- Temperatures inside delivery trucks can reach twice the recommended threshold, maybe as high as 150ºF (66ºC) in the summer, according to drivers.
- Extreme temperatures can alter the components in medications, from pancreatic enzymes to the thyroid replacement drug levothyroxine to oral contraceptives, medical experts say. For example, Toujeo, a medication used to treat Type 2 diabetes, is intended to be stored at temperatures below 86º F (30ºC).
- Extreme temperature excursions can be an issue in the winter months as well. In one case, a mail-order pack of insulin caught in a snowstorm had most likely frozen and then thawed, damaging the insulin.
- In a study published in 2023, independent pharmaceutical researchers who embedded data-logging thermometers inside simulated shipments found that the packages had spent more than two-thirds of their transit time outside the appropriate temperature range.
- Previous attempts to track temperatures using simple strips were frustrated by the incidence of false positives.
- US federal rules on drug storage conditions do not apply to the booming world of medication delivery. The US Food and Drug Administration provides strict guidelines for packaging and storing drugs and transporting them between manufacturers, wholesalers and pharmacies, but the rules do not apply to transportation to patients, which falls under the jurisdiction of states.
- Some states such as Oklahoma have attempted to tighten up regulations on shipping and packaging, but these have not yet been put into force.
- Experts warn that the growing use of mail-order drugs, coupled with rising global temperatures, could lead to significant health crises, particularly if no action is taken to enforce better handling standards.
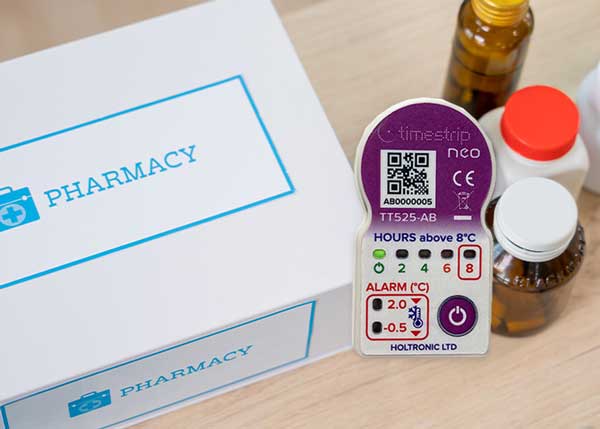
Timestrip temperature indicators for medicine shipments
The good news is that our latest product, the Timestrip neo, can avoid the false positives that have deterred the use of low-cost temperature indicators in some applications. Use of the light, small temperature indicators in pharma packaging provides an unambiguous check on temperature breaches.
For identifying compromised medicine shipments, a range of pharmaceutical shipping indicators is available as standard for common temperature ranges, and special ranges can be specified.
Timestrip Introduces World’s First Multiple Use Temperature Indicator

Timestrip® has introduced what is believed to be the first multiple-use temperature indicator of its kind available.
The Timestrip neo Multi is an electronic temperature and time indicator that can be used up to five times before being replaced.
The resettable feature significantly reduces the cost per use as well as simplifying inventory control. By avoiding the need to dispose of indicators after each use, it also represents a move towards sustainability, reducing waste by up to 80%.
Resettable temperature and time indicators
Early adopters of the neo Multi include the ‘food served on the move’ sector, i.e. air, road and rail operators who need to monitor the storage conditions of food to be served in transit. By tracking the conditions experienced by products at unit or pack level, passenger safety can be safeguarded, and unnecessary food waste avoided.
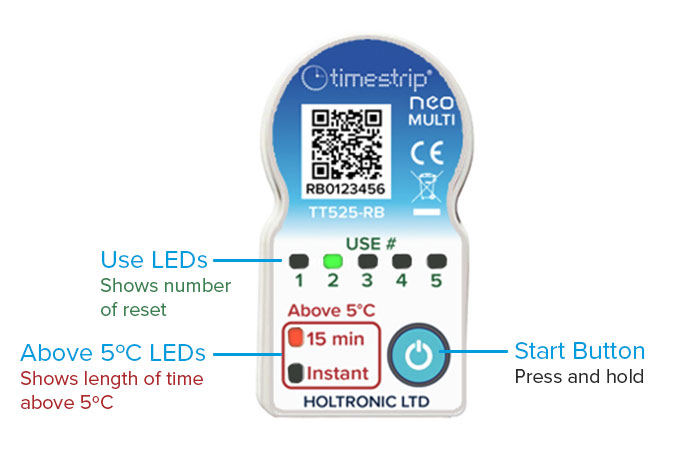
Customers have a small reset device into which the neo indicator clips, and with a single button press, the indicator is ready for its next use. The number of uses is shown on the product by a set of LEDs. Accidental resetting or tampering with the indicator are avoided by requiring use of the special reset device.
In common with the rest of the neo range, the neo Multi is robust and very small and lightweight, making it suitable for attaching even to small products. Scanning a QR code on the face of the neo Multi allows access to the individual serial number record for full auditability.
“Following the enthusiastic reception for the Timestrip neo range after its introduction eighteen months ago, we are delighted to introduce this further example of technology innovation. We expect strong interest from sectors including hospitals and pharmacies as well as food and beverage.”
Gabriel McGlynn, Chairman of Timestrip

Custom variants of the neo Multi can be specified to address specific challenges in temperature and time monitoring. Contact us about multi use temperature monitoring today.
As the seasons change, temperature monitoring becomes a top priority for organizations across various industries. Whether you're in food and beverage, pharmaceutical, healthcare, or logistics, ensuring your products remain at the right temperature is crucial.
Chill Out with our Exclusive Freeze Alarm Offer – October 2023 only
We want as many people to try the Freeze Alarm as possible, so for the entire month of October, we're excited to offer the Timestrip neo Freeze Alarm at an amazing discount of nearly 60%!
We believe this is the most cost-effective temperature indicator of its kind on the market.
Don't miss out on this fantastic opportunity to experience the benefits of Timestrip neo for your business.

Timestrip neo Freeze Alarm – Your Temperature Guardian!
The Timestrip neo Freeze Alarm is an electronic temperature indicator that provides accurate monitoring and monitors freeze events that occur during shipment.
Get your Freeze Alarm offer today!
In a significant stride towards enhancing its supply chain and ensuring the integrity of 3D printer powder supply, A prominent global technology leader has adopted Timestrip neo indicators as a temperature monitor for shipping.
Global Tech Leader Adopts Timestrip neo for Critical Temperature Monitoring
These cutting-edge indicators have emerged as the preferred choice for real-time temperature monitoring during the shipping process, , safeguarding the consumable's quality throughout its journey..
At the heart of this breakthrough solution are Timestrip's bespoke Timestrip neo TT525 indicators, meticulously designed to offer continuous temperature tracking capabilities. This seamless monitoring mechanism acts as a robust shield, upholding the consumable's optimal quality from the point of origin to its final destination.
What sets this innovative temperature monitoring solution apart is its utilization of irreversible electronic indicators. By employing these indicators, the technology leader underscores its unwavering dedication to delivering products of unparalleled quality.
The integration of Timestrip's neo indicators into the shipping process has resulted in a comprehensive quality assurance framework.

As the 3D printer powder embarks on its expedition to various destinations, the Timestrip neo indicators stand as proof of temperature controlled shipping at every step of the way.
Throughout the journey, these indicators dutifully monitor for deviations in temperature, ensuring transparency and accountability in handling and storage conditions. Timestrip neo indicators will show, via flashing LED lights, when 3D printer powder has been exposed to extreme temperatures in transit.
This invaluable information empowers the end user with confidence, knowing that their 3D printer powder has traversed its voyage under the most favorable conditions.
Read more about 3D printer powder temperature storage and shipping
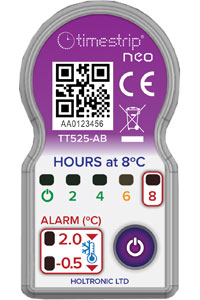
Timestrip® has developed a completely new electronic indicator technology capable of highly versatile operation at low cost. The Timestrip neo is a platform for a series of single use electronic temperature indicators suitable for pack level monitoring with both time and multiple temperature settings.
Timestrip neo
The Timestrip neo can be specified in a wide range of temperatures for both ascending and descending alerts, and for different time durations. If any of the set conditions are breached, a system of flashing LEDs provides clear information to the user. A standard configuration is available from stock.
The small form factor of the micro-weight Timestrip neo, and its ease of use, makes it ideal for widespread use on packages in the pharmaceutical, healthcare, food and high value manufacturing sectors.
Time and temperature integration is also supported, so that acceptable time limits are reduced at elevated temperatures, making the Timestrip neo ideal for protecting goods such as perishable foodstuffs that deteriorate faster at higher temperatures.
The unique design provides monitoring at three temperatures, and shows the cumulative time above a specific temperature level. For example, in the standard configuration, a descending temperature that reaches 2⁰C will cause an LED to flash; another LED will light when this drops as low as -0.5⁰C. A third set of LEDs show when the temperature has exceeded 8⁰C for 2, 4, 6 or 8 hours.
A unique serial number is provided on every unit to provide traceability as part of a quality control system.
Timestrip neo technology opens up a new avenue of possibilities for Timestrip. Partnering with electronic design and manufacturing specialists Holtronic Ltd to design the Timestrip neo platform has enabled us to offer a completely new series of products to our clients.
Gabriel McGlynn, Chairman of Timestrip
“The Timestrip neo platform technology is complementary to liquid-based indicators, and offers higher accuracy while maintaining the low cost, small form solutions our customers need”, said Nora Murphy, Commercial Director of Holtronic. “The small size and cost effectiveness of Timestrip neo are common benefits across the series of products.”
Want to see more about our newest indicator? Read more about electronic time and temperature monitoring here.
Samples of Timestrip neo are available mid-August, product available from mid-September.
The New Way of Reading Logged Temperature Data
Timestrip® has introduced its first temperature data logger – the TL520. This compact and economical ‘micro data logger’ provides alerts when temperatures breach set limits, and allows the logged data to be downloaded to an app for investigation and analysis.
Simpler and easier to use than conventional data loggers, the TL520 requires minimal training in use, and can be used at any point in the cold chain.
The TL520 is a single use device providing an immediate irreversible indication of over- and under-temperature conditions using built-in LED lights. Accuracy is quoted as ±0.5ºC over the normal operating range.
A novel feature of the TL520 is that stored data can be sent wirelessly to an accompanying app. for investigation and analysis using built-in NFC technology. No computer is required as the data downloads to any compatible mobile device, and both iOS and Android devices are supported.
The 2-8ºC default settings make the TL520 ideal for monitoring cold chain (refrigerated) temperatures in the storage and transport of sensitive products. Pharmaceuticals, blood, vaccines and food products are typical end use applications.
The new micro logger provides many of the benefits of bulky, traditional data loggers, yet is much simpler to use and is small enough to store with many products during transport and storage
Nora Murphy, Commercial Director of Timestrip
The TL520 is now available from Timestrip and its distributors.
Timestrip smart indicators were used in a study by academics at Reading School of Pharmacy, part of The University of Reading, into people’s confidence in the quality of medicines.
The study focused on the potential re-use of returned medicines. It found that participants’ intention to take part in medicines re-use increased with the presence of the Timestrip® Plus temperature sensors on the packaging. The perceived social pressure to accept the medication also increased. The effect of the indicators was even greater than the promise of pharmacist visual checking of the medicines.
Although medicines re-use is not at present allowed in the UK, the presence of Timestrip indicators on packaging is understood to be a marker of their quality.
Ref: Lam, Y.; McCrindle, R.; Hui, T.K.L.; Sherratt, S.; Donyai, P. The Effect of Quality Indicators on Beliefs about Medicines Reuse: An Experimental Study. Pharmacy 2021, 9, x. https://doi.org/10.3390/xxxxx. Read more here: https://www.mdpi.com/2226-4787/9/3/128
Timestrip® has introduced new time indicators to support the storage and handling of coronavirus vaccine supplies. These smart single use indicators provide an irreversible alert if the vaccine is stored in refrigerated conditions (2-8°C) for too long after thawing from deep freeze storage.
In an early case example, now that the Pfizer / Biontech vaccine has been approved for UK use, attention has turned to the logistics of supplying it to huge numbers of people. Timestrip indicators can travel with the vaccine supply and withstand the coldest storage temperatures e.g. down to -70°C, yet react when the vaccine consignment is thawed.
From the Timestrip vaccine range, the VRM30 indicator shows the time that vaccines have been held in the chilled 2-8°C range, and has markers for 1, 5 days up to 30 days, making it a suitable solution for the management of many vaccines including from Pfizer, Moderna / J&J and AstraZeneca. VRM30 is designed to compensate automatically for changes in temperature, responding more quickly in warmer conditions.
When the vaccine has been removed from the fridge into room temperature ready to be administered, a VOR12 indicator can be deployed to alert logistics and healthcare professionals that an 8°C threshold has been breached.
It will show the duration for which the vaccine has been Out of Refrigerator, as the indicator window gradually fills. A completely full window indicates that the maximum allowed storage period has been reached, and the vaccine may be compromised.
The eTimestrip range of electronic indicators comprises single use devices that generate a report via a mobile app, and provide detailed information on time and temperature to responsible healthcare staff. This report can be sent for centralised storage, eliminating the need to return the device itself for data analysis.
Conventionally, data loggers can be used in these applications, but the scale of the current challenge means that Timestrip’s small, robust indicators are a lower cost, practical solution, while supporting compliance with regulations.
We continue to innovate at a rapid pace to provide cost effective monitoring solutions, considering the efforts of the WHO, FDA and government bodies in their respective countries. Our main objective is supporting healthcare teams as they provide safe care to nations worldwide.
Nora Murphy, Commercial Director
Timestrip®, the developer of smart indicator technology, has released two new ranges designed to be used with the latest coronavirus vaccines now becoming available. The indicators track temperature and time, and will ensure that vaccines are fresh when administered to patients.
The Timestrip Vaccine Thaw Timer is an event thaw indicator with 24 hour time indication calibrated to run at fridge temperatures. It can be packed with vaccines that require storage at -70°C in the ultra-cold chain, and automatically starts monitoring as the vaccine is thawed and transferred to chilled conditions at the hospital or surgery. It then tracks the time from thaw at 6 hours, 12 hours and up to 24 hours, meeting the specifications for use from manufacturers such as Pfizer.
The Timestrip Vaccine Refrigeration Monitor range comprises three indicators. One is designed to indicate the thawing event of the vaccine; another will time 30 days duration in refrigerator conditions (2°C-8°C), helping the user to manage the vaccine manufacturer’s guidelines for use.
Then, when the vaccine has been taken from the refrigerator and prepared for use, a third indicator monitors how long it has been exposed to temperatures above 8°C, typically at room temperature. The time ‘run out’ for the Vaccine Refrigeration Monitor VOR12 (Vaccine Out of Refrigerator) is up to 12 hours, with shorter times to an alert being seen at higher temperatures.
These Vaccine Refrigeration Monitor products will help overcome challenges in the handling of COVID-19 vaccines up to the point of administering, and are designed to support the manufacturers’ specification for the handling of vaccines such as those from Moderna.
Both indicators provide a clear irreversible indication to healthcare staff that conditions of storage and handling have exceeded acceptable conditions, and that the vaccine consignment cannot be relied upon.
Based on safe chemical technology, both the Vaccine Thaw Timer and Vaccine Refrigeration Monitor range overcome the limitations of batteries used at ultra-low temperatures.
We continue to innovate our patented technology to bring the benefits to important applications such as vaccine distribution, supply and last mile handling. Using these indicators, healthcare professionals worldwide can be supported cost effectively, and guided through soon to be routine practices of handling and managing these vital resources.
Nora Murphy, Commercial Director of Timestrip.
Timestrip has recently added other products to its range in response to coronavirus. These include an indicator to monitor antiviral solutions, and Rapid Diagnostics 15, a 15 minute timer indicator for use with rapid turnaround lateral flow test kits.
Timestrip has a new white paper detailing the importance of monitoring time and temperature parameters during storing and transporting to point of care
For more information on how Timestrip UK Ltd is handling the COVID-19 pandemic, please see this post.