Medicines exposed to the wrong conditions – such as the high temperatures now found in transporting vehicles - can be damaged and endanger health. This excellent piece from the New York Times explains the problem (ten minute read).
Don’t have ten minutes? Read this two minute version instead:
Top pharmaceutical temperature shipment takeaways
- Doctors and pharmacists say the scorching temperatures enveloping the country could be endangering people’s health in an unexpected way: by overheating their medications.
- Millions of Americans now receive their prescription medications through mail-order shipments, and this method is increasingly required by health insurance plans.
- Temperatures inside delivery trucks can reach twice the recommended threshold, maybe as high as 150ºF (66ºC) in the summer, according to drivers.
- Extreme temperatures can alter the components in medications, from pancreatic enzymes to the thyroid replacement drug levothyroxine to oral contraceptives, medical experts say. For example, Toujeo, a medication used to treat Type 2 diabetes, is intended to be stored at temperatures below 86º F (30ºC).
- Extreme temperature excursions can be an issue in the winter months as well. In one case, a mail-order pack of insulin caught in a snowstorm had most likely frozen and then thawed, damaging the insulin.
- In a study published in 2023, independent pharmaceutical researchers who embedded data-logging thermometers inside simulated shipments found that the packages had spent more than two-thirds of their transit time outside the appropriate temperature range.
- Previous attempts to track temperatures using simple strips were frustrated by the incidence of false positives.
- US federal rules on drug storage conditions do not apply to the booming world of medication delivery. The US Food and Drug Administration provides strict guidelines for packaging and storing drugs and transporting them between manufacturers, wholesalers and pharmacies, but the rules do not apply to transportation to patients, which falls under the jurisdiction of states.
- Some states such as Oklahoma have attempted to tighten up regulations on shipping and packaging, but these have not yet been put into force.
- Experts warn that the growing use of mail-order drugs, coupled with rising global temperatures, could lead to significant health crises, particularly if no action is taken to enforce better handling standards.
Timestrip temperature indicators for medicine shipments
The good news is that our latest product, the Timestrip neo, can avoid the false positives that have deterred the use of low-cost temperature indicators in some applications. Use of the light, small temperature indicators in pharma packaging provides an unambiguous check on temperature breaches.
For identifying compromised medicine shipments, a range of pharmaceutical shipping indicators is available as standard for common temperature ranges, and special ranges can be specified.

Like many hospitals, one US-based Timestrip customer maintains a fleet of ambulances that serves the local community. Use of the pharmaceutical products carried by the ambulances is subject to rules laid down by the state Board of Pharmacy.
This is responsible for regulating the practice of pharmacy and the legal distribution and dispensing of prescription drugs and precursor substances.
One stipulation requires hospital-based ambulance services to monitor their drug kits for extreme temperatures while out in the field. So hospital medics turned to Timestrip monitoring devices to track the kits as they are stored and transported.

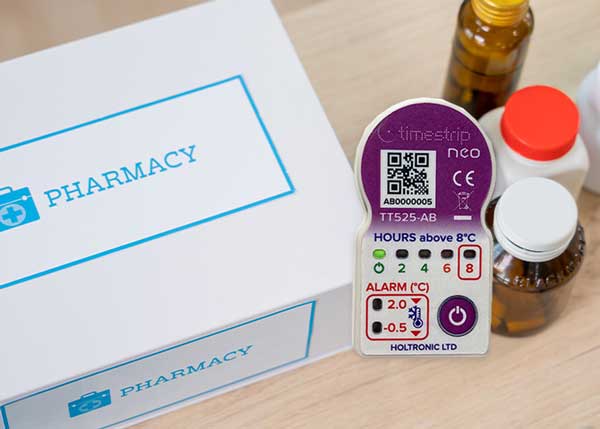
Timestrip liquid-based TTIs and Timestrip neo TT525-AB electronic indicators are placed on the drug kits before they are deployed in the field. In this way, the hospital receives a clear, irreversible visual alert if the drugs are exposed to conditions below freezing point or above 100ºF (48ºC).
Timestrip neo is a range of very small and light time and temperature indicators that provide a highly cost effective method of monitoring the conditions in which drugs and other products are kept.
Using the tiny Timestrip devices means the hospital can be sure whether the acceptable pharmaceutical temperature excursions of products has been breached or not. This safeguards the patients and means that medicines don’t have to be discarded unnecessarily.

Pioneers Sustainable Healthcare with Innovative Medicines Returns System
Radboud University Medical Center (Radboudumc), a leading institution in sustainable and innovative healthcare, and Timestrip UK, a leading supplier of time and temperature indicators. are spearheading a groundbreaking initiative to address challenges in re-allocating medicines returned unused from patients.
A panel of 14 hospitals across The Netherlands is implementing a system involving sealed bags containing oncology medicines and Timestrip neo indicators for drugs dispensed to patients. The neo indicators were selected for their clear irreversible alerts, small size and reliability.
Currently, unused medicines returned by patients are not re-dispensed, leading to wastage and potential delays in treatment. Recognizing the need for a more sustainable approach, researchers at Radboudumc have embarked on a trial to evaluate the practicality of a returns system that ensures the authenticity and proper storage conditions of returned medicines.
The panel of 14 hospitals that have been established to participate in the trial comprises six university hospitals, six teaching hospitals and two general hospitals.
When any of the drugs used in the trial are returned to the hospital pharmacy unused, the pharmacists can check that the package has not been interfered with, and that the temperatures incurred during storage and transport have not exceeded specified limits.

A custom neo indicator was developed to monitor three oncology medicine temperature ranges:
2ºC, 25ºC and 30ºC. A series of LEDs on the front of the indicator show clearly when certain temperature limits have been breached. The indicators also show the duration of exposure to 25ºC up to eight hours.
As the neo indicator alerts are clear and irreversible, clinicians can have confidence in the readings, and a further safeguard is included in the form of a QR code on each indicator that can be scanned to provide serial number data for traceability.
Shirley Xie MSc, the Investigator for the trial at Radboudumc, explains: “Using a temperature indicator is important in providing the evidence needed during the trial. We needed a simple yet effective device which gives us a visible confirmation of medicine quality.”
The ongoing trial is expected to demonstrate the cost-effectiveness of the proposed system for re-dispensing oncology (and potentially other) medicines, thereby reducing waste.
Miss Xie emphasizes the significance of the trial: "As well as the potential improvements in efficiency and patient care from re-dispensing unused drugs of good quality, there is a sustainability benefit associated with the reduced waste."
Radboudumc remains committed to making a significant impact on health and healthcare, and this initiative aligns with their broader mission of advancing healthcare practices for the benefit of patients and the environment.
Read more about our partnership with Radboudumc on oncology medicine temperature indicators.
Timestrip will be demonstrating our healthcare time and temperature monitors this weekend.
Community and primary care pharmacy professionals will be meeting on the 15th and 16th of October at The Pharmacy Show. This years Pharmacy Show will be held at the NEC in Birmingham.
Come and meet with us - we’ll be at Stand M50. Test and touch our new products and solutions.

If you are a healthcare or pharmaceutical professional, you can get practical content such as how Timestrip time and temperature indicators can streamline your operations.
The Pharmacy Show Birmingham
The Pharmacy Show is the UK's only dedicated trade show and conference for the community and primary care pharmacy sector.
There will be over 300 exhibitors and hundreds of hours of live education content on the latest knowledge, innovations and technology in pharmacy.
Read more about The Pharmacy Show
1. Timestrip has developed innovative time and temperature indicators that travel with healthcare products and provide a clear indication of time in use, or a breach in acceptable storage or use conditions. The disposable ‘smart’ indicators provide peace of mind, and alert operators to the need for intervention.
2. Temperature and time monitoring of pharmaceutical or other sensitive products is achieved throughout the whole cold chain, from manufacturer through transport and logistics to retailer and patient.
3. Summer temperatures are an especially important time for ensuring safe storage and shipping of medicines e.g. for pharmacists using home delivery. Patients have confirmation that goods have been safely transported.
4. When applied to temperature or time sensitive products, customer benefits include reduced product wastage, audit trail / traceability, and assistance in regulatory compliance.
5. Timestrip’s full range of liquid-based indicators are capable of monitoring time from one hour to two years, and temperatures from -4°F to 100°F (-20°C to 38°C). Electronic products are available which include devices that store data for download to smartphones.
6. Vaccine storage and shipments and transport of virus specimens are just two of many applications where hospitals need reliable temperature monitoring. The TL520 is a compact and economical ‘micro data logger’ that provides alerts when temperatures pass set limits, and provides an immediate irreversible indication of over- and under-temperature conditions using built-in LED lights.
7. The TL520 is a single use device with an accuracy of ±0.5ºC over the normal operating range. It requires minimal training in use, and can be used at any point in the cold chain.
8. Timestrip is a global company with 15 years’ experience designing and manufacturing unique products. It services customers around the world, direct and through a network of specially trained distributors.
9. The company’s own research and development team develops new products for bespoke applications. It has an IQC–approved certified manufacturing facility that conforms to ISO 13485:2016, and ISO 9001:2015. Regulatory compliance: Timestrip has a FDA 510K medical device for blood bag temperature monitoring.
10. Smart indicators from Timestrip provide peace of mind for healthcare professionals and patients alike.
Want to know more about Timestrip time temperature technology
Interested in how you can incorporate Timestrip into your monitoring? View our pharmaceutical solutions. For enquiries, please contact us.
Timestrip will be attending the 7th Pharma Supply Chain & Logistics Innovation Programme in Basel on 10-11 May 2022. We will be at Booth #11. Nora Murphy and Roger Sabat will give a brief presentation on ‘New Challenges and Solutions in Temperature Monitoring’.
At the conference, Timestrip will show a new electronic indicator, designed to give low cost, easy to use, effective monitoring.

We will also be demonstrating other products from our range of irreversible temperature indicator products for the pharmaceutical industry. These include our Timestrip PLUS irreversible ascending temperature indicators, Electronic Timestrip Complete indicators and Timestrip Micro Logger for 2-8ºC shipping.
Our small team at Booth #11 will be delighted to meet up to discuss your requirements, so please come and get a sample of our Irreversible Temperature Indicators to test and take away with you. Click here for more information about our Timestrip PLUS indicators and here for information about our electronic indicators.
Timestrip temperature monitoring labels make tracking temperature breaches across a multitude of cold chain pharmaceutical applications a simple, cost-effective process.
In fact, we offer some of the most cost-effective solutions of our type in Pharma logistics, which is why our technology has already been adopted by a number of innovative businesses. Our precise, efficient, user-friendly temperature indicator labels are helping to ensure effective cold chain management around the world.
If you are planning on attending PSIP in Basel, please get in touch to arrange a meeting with us.
Timestrip smart indicators were used in a study by academics at Reading School of Pharmacy, part of The University of Reading, into people’s confidence in the quality of medicines.
The study focused on the potential re-use of returned medicines. It found that participants’ intention to take part in medicines re-use increased with the presence of the Timestrip® Plus temperature sensors on the packaging. The perceived social pressure to accept the medication also increased. The effect of the indicators was even greater than the promise of pharmacist visual checking of the medicines.
Although medicines re-use is not at present allowed in the UK, the presence of Timestrip indicators on packaging is understood to be a marker of their quality.
Ref: Lam, Y.; McCrindle, R.; Hui, T.K.L.; Sherratt, S.; Donyai, P. The Effect of Quality Indicators on Beliefs about Medicines Reuse: An Experimental Study. Pharmacy 2021, 9, x. https://doi.org/10.3390/xxxxx. Read more here: https://www.mdpi.com/2226-4787/9/3/128
Timestrip®, the developer of smart indicator technology, has released two new ranges designed to be used with the latest coronavirus vaccines now becoming available. The indicators track temperature and time, and will ensure that vaccines are fresh when administered to patients.
The Timestrip Vaccine Thaw Timer is an event thaw indicator with 24 hour time indication calibrated to run at fridge temperatures. It can be packed with vaccines that require storage at -70°C in the ultra-cold chain, and automatically starts monitoring as the vaccine is thawed and transferred to chilled conditions at the hospital or surgery. It then tracks the time from thaw at 6 hours, 12 hours and up to 24 hours, meeting the specifications for use from manufacturers such as Pfizer.
The Timestrip Vaccine Refrigeration Monitor range comprises three indicators. One is designed to indicate the thawing event of the vaccine; another will time 30 days duration in refrigerator conditions (2°C-8°C), helping the user to manage the vaccine manufacturer’s guidelines for use.
Then, when the vaccine has been taken from the refrigerator and prepared for use, a third indicator monitors how long it has been exposed to temperatures above 8°C, typically at room temperature. The time ‘run out’ for the Vaccine Refrigeration Monitor VOR12 (Vaccine Out of Refrigerator) is up to 12 hours, with shorter times to an alert being seen at higher temperatures.
These Vaccine Refrigeration Monitor products will help overcome challenges in the handling of COVID-19 vaccines up to the point of administering, and are designed to support the manufacturers’ specification for the handling of vaccines such as those from Moderna.
Both indicators provide a clear irreversible indication to healthcare staff that conditions of storage and handling have exceeded acceptable conditions, and that the vaccine consignment cannot be relied upon.
Based on safe chemical technology, both the Vaccine Thaw Timer and Vaccine Refrigeration Monitor range overcome the limitations of batteries used at ultra-low temperatures.
We continue to innovate our patented technology to bring the benefits to important applications such as vaccine distribution, supply and last mile handling. Using these indicators, healthcare professionals worldwide can be supported cost effectively, and guided through soon to be routine practices of handling and managing these vital resources.
Nora Murphy, Commercial Director of Timestrip.
Timestrip has recently added other products to its range in response to coronavirus. These include an indicator to monitor antiviral solutions, and Rapid Diagnostics 15, a 15 minute timer indicator for use with rapid turnaround lateral flow test kits.
Timestrip has a new white paper detailing the importance of monitoring time and temperature parameters during storing and transporting to point of care
For more information on how Timestrip UK Ltd is handling the COVID-19 pandemic, please see this post.
An FDA-approved Irreversible Time and Temperature Indicator that ensures that only viable whole blood supplies are used post-transit
The high tech, low cost alternative to electronic dataloggers
|
A critical issue for medical professionals is ensuring the viability of blood supplies once they leave the highly controlled storage environment, such as a blood bank refrigerator or a hospital blood storage facility. This aspect of the so-called blood cold chain is essential for ensuring that these are kept at the correct temperature right up to the time they are administered by trauma units, ERs, operating theatre staff, air/ ground ambulances, and any other situation requiring blood to be transported from storage units out to patients.
During transit, whole blood needs to be kept within a temperature range of 2°C/36OF to 10°C/50°F. Below this range, there is a risk of freezing with the formation of ice crystals, rendering the blood unusable, while upper temperature breaches create the possibility of blood being affected by an overgrowth of non-specific bacteria, which may have entered the blood unit during collection or component preparation.
Both these situations have serious consequences: the cost implications of a wasted product and/or more seriously, a patient that is put at risk.
To address these issues, Timestrip® has developed its single-use Blood Temp 10 – a high-tech, low cost Irreversible Time & Temperature Indicator that effectively monitors temperature breaches above 10°C/50°F during transit.
No more guesswork
Thanks to its innovative technology, Timestrip® Blood Temp 10 removes this uncertainty by providing clear, unambiguous data as to whether a supply of whole blood is safe to use or be returned to storage.
This is achieved thanks to the smart label’s two indicator windows:
• the first window to indicate if the label has been activated
• the second to indicate if a temperature breach has occurred
Thus, at a glance, medical staff know if a supply of whole blood is not only safe to use post-transit. The smart label also ensures a higher level of quality of the product is delivered by monitoring of temperature throughout transit.
Moreover, thanks to its irreversible feature, once the smart label has reacted to a temperature breach, this cannot be undone. For security reasons, the colour of the label’s indicator window stays permanently changed.
FDA approved … and more
Timestrip® Blood Temp 10 has FDA certification (FDA 510(k) #BK190363) and is manufactured with controls established by a IQC approved quality management system that conforms with ISO 9001:2015 and ISO 13485:2016.
With a temperature accuracy of +/- 0.5°C, this means that the Timestrip® label is designed with reliable and, more importantly, trustworthy technology as assessed by some of the world’s most stringent criteria. Moreover, extra security is assured by every Timestrip® label having its own unique serial number, thus ensuring full traceability.
Checking this list of features brings
further benefits to healthcare providers involved in the transportation of
whole blood by also ticking the box for local and internal regulatory
compliance.
Cost savings
Because Timestrip® Blood Temp 10 ensures that only viable blood supplies are either administered post-transit, or returned to storage, the possibility of precautionary disposal is eliminated. This reduction in waste can have significant cost benefits.
In fact, studies have shown that loss rates without a Timestrip® are around 5%. These are halved with its use. In fact, reducing a loss rate by 9.9% pays for the total investment and a conservative 19.8% reduction doubles the ROI.
The generation of similar positive temperature monitoring outcomes are possible using other products, such as electronic devices. These, however, are far costlier.
Ease of use
Apart from delivering clear, unambiguous data as to whether post-transit a unit of whole blood may be used or returned to storage thanks its innovative indicator windows, the Timestrip® Blood Temp 10 is also remarkably easy to use.
In fact, unlike other whole blood temperature monitoring solutions on the market, Timestrip® Blood Temp 10 is a unique FDA 510(k) medical device that does not require preconditioning. Moreover, it is also CE approved and designed for:
• quick and easy application
• no prior conditioning by blood storage staff
• temperature breach data delivered at a glance by medical field personnel.
Prior to the transit of a unit of whole blood, a simple squeeze of the label’s activation blister is all that is required for temperature monitoring of a unit of whole blood to begin. Then the label just needs to stuck on the unit.
Squeeze, peel and stick – that’s it!
And the end of transit, Timestrip® technology has also been designed for maximum ease of use:
• until activation, the first indicator window is yellow and the second is white
• upon activation, the first indicator window turns green
• if a temperature breach has occurred during transit, the duration of the breach is indicated by the second white indicator window that proportionately fills up with blue dye
Product Specifications
| 10°C/50°F monitoring |
| Irreversible, single use |
| 19mm x 32mm |
| Field activation |
| Visual proof of temperature exposure |
Advantages over electronic dataloggers
Beyond being less expensive, Timestrip® Blood Temp 10 does not involve a datalogger’s complicated and sometimes cumbersome unit return program that is necessary to make it more affordable. The smart label’s single use indicator makes it the ideal fit for simple, straightforward and effective temperature monitoring.
The Timestrip® also is a super convenient shape: small, flat, with no batteries or any other electronic components to them.
For green credentials, the Timestrip® also outstrips traditional dataloggers in terms of carbon footprint, because the latter eventually needs to be either be thrown away or sent back to source to justify the expenditure.
Lastly, the Timestrip® has the potential of generating more accurate safety data. Indeed, because a container of units of whole blood will tend to use just a single datalogger, inevitably the temperature of ambient air within the container is taken into account, potentially generating less than accurate safety data.
With Timestrip® labels however, every single unit has its own temperature indicator. This means that the possibility of rejecting an entire shipment based on a single result is eliminated.