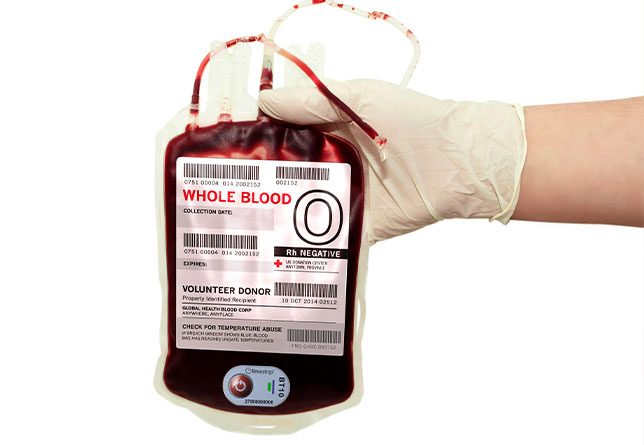
Timestrip Blood Temp 10: less waste, lower cost, less risk
An FDA-approved Irreversible Time and Temperature Indicator that ensures that only viable whole blood supplies are used post-transit
The high tech, low cost alternative to electronic dataloggers
|
A critical issue for medical professionals is ensuring the viability of blood supplies once they leave the highly controlled storage environment, such as a blood bank refrigerator or a hospital blood storage facility. This aspect of the so-called blood cold chain is essential for ensuring that these are kept at the correct temperature right up to the time they are administered by trauma units, ERs, operating theatre staff, air/ ground ambulances, and any other situation requiring blood to be transported from storage units out to patients.
During transit, whole blood needs to be kept within a temperature range of 2°C/36OF to 10°C/50°F. Below this range, there is a risk of freezing with the formation of ice crystals, rendering the blood unusable, while upper temperature breaches create the possibility of blood being affected by an overgrowth of non-specific bacteria, which may have entered the blood unit during collection or component preparation.
Both these situations have serious consequences: the cost implications of a wasted product and/or more seriously, a patient that is put at risk.
To address these issues, Timestrip® has developed its single-use Blood Temp 10 – a high-tech, low cost Irreversible Time & Temperature Indicator that effectively monitors temperature breaches above 10°C/50°F during transit.
No more guesswork
Thanks to its innovative technology, Timestrip® Blood Temp 10 removes this uncertainty by providing clear, unambiguous data as to whether a supply of whole blood is safe to use or be returned to storage.
This is achieved thanks to the smart label’s two indicator windows:
• the first window to indicate if the label has been activated
• the second to indicate if a temperature breach has occurred
Thus, at a glance, medical staff know if a supply of whole blood is not only safe to use post-transit. The smart label also ensures a higher level of quality of the product is delivered by monitoring of temperature throughout transit.
Moreover, thanks to its irreversible feature, once the smart label has reacted to a temperature breach, this cannot be undone. For security reasons, the colour of the label’s indicator window stays permanently changed.
FDA approved … and more
Timestrip® Blood Temp 10 has FDA certification (FDA 510(k) #BK190363) and is manufactured with controls established by a IQC approved quality management system that conforms with ISO 9001:2015 and ISO 13485:2016.
With a temperature accuracy of +/- 0.5°C, this means that the Timestrip® label is designed with reliable and, more importantly, trustworthy technology as assessed by some of the world’s most stringent criteria. Moreover, extra security is assured by every Timestrip® label having its own unique serial number, thus ensuring full traceability.
Checking this list of features brings
further benefits to healthcare providers involved in the transportation of
whole blood by also ticking the box for local and internal regulatory
compliance.
Cost savings
Because Timestrip® Blood Temp 10 ensures that only viable blood supplies are either administered post-transit, or returned to storage, the possibility of precautionary disposal is eliminated. This reduction in waste can have significant cost benefits.
In fact, studies have shown that loss rates without a Timestrip® are around 5%. These are halved with its use. In fact, reducing a loss rate by 9.9% pays for the total investment and a conservative 19.8% reduction doubles the ROI.
The generation of similar positive temperature monitoring outcomes are possible using other products, such as electronic devices. These, however, are far costlier.
Ease of use
Apart from delivering clear, unambiguous data as to whether post-transit a unit of whole blood may be used or returned to storage thanks its innovative indicator windows, the Timestrip® Blood Temp 10 is also remarkably easy to use.
In fact, unlike other whole blood temperature monitoring solutions on the market, Timestrip® Blood Temp 10 is a unique FDA 510(k) medical device that does not require preconditioning. Moreover, it is also CE approved and designed for:
• quick and easy application
• no prior conditioning by blood storage staff
• temperature breach data delivered at a glance by medical field personnel.
Prior to the transit of a unit of whole blood, a simple squeeze of the label’s activation blister is all that is required for temperature monitoring of a unit of whole blood to begin. Then the label just needs to stuck on the unit.
Squeeze, peel and stick – that’s it!
And the end of transit, Timestrip® technology has also been designed for maximum ease of use:
• until activation, the first indicator window is yellow and the second is white
• upon activation, the first indicator window turns green
• if a temperature breach has occurred during transit, the duration of the breach is indicated by the second white indicator window that proportionately fills up with blue dye
Product Specifications
| 10°C/50°F monitoring |
| Irreversible, single use |
| 19mm x 32mm |
| Field activation |
| Visual proof of temperature exposure |
Advantages over electronic dataloggers
Beyond being less expensive, Timestrip® Blood Temp 10 does not involve a datalogger’s complicated and sometimes cumbersome unit return program that is necessary to make it more affordable. The smart label’s single use indicator makes it the ideal fit for simple, straightforward and effective temperature monitoring.
The Timestrip® also is a super convenient shape: small, flat, with no batteries or any other electronic components to them.
For green credentials, the Timestrip® also outstrips traditional dataloggers in terms of carbon footprint, because the latter eventually needs to be either be thrown away or sent back to source to justify the expenditure.
Lastly, the Timestrip® has the potential of generating more accurate safety data. Indeed, because a container of units of whole blood will tend to use just a single datalogger, inevitably the temperature of ambient air within the container is taken into account, potentially generating less than accurate safety data.
With Timestrip® labels however, every single unit has its own temperature indicator. This means that the possibility of rejecting an entire shipment based on a single result is eliminated.







