Timestrip will be attending the 29th Regional Congress of the International Society of Blood Transfusion (ISBT) in Basel, Switzerland from 22nd - 26th June 2019
See our blood temp indicators
We will demonstrate our Timestrip® Blood Temp 10 and Timestrip® Blood Temp 6 at the exhibition. We will be at Booth U106, Hall 4.U, please drop by and get a sample of our Blood Temp indicators to take away with you.
Our Blood Temp irreversible blood bag temperature indicators monitor temperature compliance in blood bags. They are especially relevant for monitoring blood temperature during transit from the highly controlled environment of the blood bank to use or store in a different destination, such as a hospital.
ISBT is a society in the field of blood transfusion, transplants, science and medicine. It facilitates knowledge about transfusion medicine to serve the interests of donors and patients.
Whether in a major teaching hospital, a walk-in clinic or a doctor’s surgery, any type of healthcare boils down to two broad objectives: improved patient outcomes and the best possible use of resources.
Although historically reconciling patient health and the harsh realities of budgetary constraints have at times led to difficult choices, the fact is that by integrating technology and innovation into decision making around resource allocation, both objectives can be achieved at the same time.
Indeed, thanks to its high tech / low cost Time and Temperature Indicator labels, Timestrip® is able to provide healthcare providers and the pharmaceutical sector with a broad range of practical, user-friendly solutions that:
✔ Ensure product quality
✔ Reduce product waste
✔ Ensure regulatory compliance
✔ Reduce time and temperature monitoring costs
But what does this look like in practice?
Improving quality control and efficiency

A bag of whole blood with a Timestrip® Blood Temp 10°C
Tenet Healthcare is US healthcare provider that faced the challenge of needing to effectively manage how it processed bags of whole blood. On the one hand, it faced the need to comply with local regulatory requirements related to the use of effective temperature monitoring for blood products; and on the other, it had to discard any bag of blood that had been exposed to a temperature above 10°C for more than 30 minutes.
The company needed to be absolutely certain that any unused bag of whole blood was 100% safe to be returned to storage for future use. It had also noted ongoing difficulties in using other blood temperature indicators. Potentially, the latter could lead to doubts about product safety, which in turn would require that bag of blood to be discarded, possibly unnecessarily.
Its use of Timestrip® Blood Temp 10 – 10°C Blood Irreversible Transit Temperature Indicator noted the following advantages:
✔ Improved quality of care
✔ Improved workflow efficiency
✔ Maintenance of quality control during transportation and storage
✔ Support in complying with regulatory guidelines
Cost-effective regulatory compliance

Marathon Distributors is a pharmaceutical and healthcare logistics services provider in Cyprus and is involved in the distribution of pharmaceutical products throughout the island. The main cold chain management issue it faced was around compliance with local and EU regulations, specifically the country’s Law on Human Pharmaceuticals 70(I) 2001 and the EU guidelines on Good Distribution & Manufacturing Practices. These mandated the company to ensure that the pharmaceuticals it delivered were not exposed to a temperature above 8°C for more than 8 hours during transportation.
Although other products helped achieve regulatory compliance, the company began using Timestrip® PLUS™ 8°C 8 Hrs (TP065) Irreversible Time and Temperature Indicators because they were much more competitively priced.
Overall the company reported:
✔ Timestrip Indicators were 50% cheaper
✔ Delivery costs were 50% lower
✔ Delivery times were faster
✔ Positive end-user feedback around ease of use
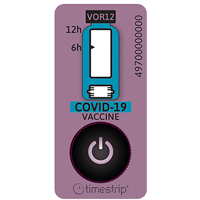
Easy temperature monitoring for vaccine transport
Cambridgeshire Community Service NHS Trust is a UK healthcare provider in south-east England. Part of its work is the delivery of school-age immunisations to schools in its area. This includes for instance flu nasal immunisations for primary schools and HPV vaccinations and Meningitis ACWY and TdIPV for secondary schools.
For the delivery of vaccines, electric Labcold Portable Vaccine Carriers and pharmaceutical-grade vaccine carriers are used. In order to maintain the efficacy of the various transported vaccines, two different temperature ranges need to be managed: 2°C to 8°C and -25°C to 25°C.
In order to ensure the delivery of viable, unspoiled vaccines, the Trust thus had a requirement for a dual, low-cost solution for use by its delivery teams.

To achieve this, two Timestrip products have been used in each vaccine carrier:
● a Timestrip® PLUS (TP 065) to indicate if the temperature went above 8°C
● a Timestrip® PLUS (TP 217) to indicate if the temperature exceeded 25°C, and if so, for how long
The feedback from the delivery teams has been extremely positive, specifically around:
✔ Easy to read visual data
✔ Ease of use
✔ Reliability
Reliable test kit time data
Clinical Innovations is a US maker of obstetrics devices that wanted to develop a non-invasive test kit for the diagnosis of any rupture of membranes (ROM). Although treatable, spontaneous ROM is a serious medical condition and a major cause of preterm births. Thus, an accurate diagnosis of ROM can be a key factor in determining timely and appropriate medical intervention. Similarly, a false diagnosis of ROM can lead to unnecessary hospitalisation, medication and even induced premature delivery.
Timestrip was able to provide Clinical Innovations with Time Indicators for its ROM Plus® self-contained test kit. These give clinicians the essential and immediate data they need to reduce the dangers of any premature ROM for expectant mothers and their babies.

The test kit uses Timestrip technology with an in-built 20-minute time monitoring indicator that generates the required data within 5-20 minutes, with 5,10 and 20 minutes clearly indicated on the Timestrip. Its key benefits are:
✔ Clear visual data
✔ Ease of use
✔ Improved health outcomes
Hygienic healthcare environment
Beyond pharmaceuticals and blood products, healthcare also involves various types of disposable items that need replacing regularly. Especially for the sick, hygiene in the clinical environment is an essential aspect of patients being able to make a full recovery. Timestrip indicators make excellent cleaning reminders for surfaces, filters and instruments.
Assuming the absence of any complications, catheters for instance need to be replaced every 30 days for Foley catheters and every 60-90 days for silicone catheters. Catheter bags however need to be replaced every 5-7 days. If these items are not replaced at appropriate intervals, opportunities are created for harmful pathogens to develop.

Unsurprisingly, this has also been shown to be the case for hospital privacy curtains. Dr Kevin Shek was the lead study author into the rate of contamination of hospital privacy curtains in a burns/plastic ward. In a report about the study findings in Nursing Times, he said:
“We know that privacy curtains pose a high risk for cross-contamination because they are frequently touched but infrequently changed. The high rate of contamination that we saw by the fourteenth day may represent an opportune time to intervene, either by cleaning or replacing the curtains."
Especially in busy hospital departments such as Accident & Emergency and ICU, hospital privacy curtains can quickly become contaminated with bacteria such as methicillin-resistant S. aureus (MRSA) and Clostridium difficile (C. diff). Thanks to Timestrip® Time Monitoring Indicator panels that change color after a set period of time, clinical staff are alerted when a privacy curtain needs to be changed. They help a hospital achieve:
✔ Best use of resources with a low-cost solution
✔ Compliance with guidelines around frequency of change
✔ Less time needed to monitor when that change needs to occur
✔ Hygienically clean hospital environment for patients
Tenet Healthcare is a healthcare services company is using Blood Temp 10 temperature indicators to monitor blood transportation. PDC Healthcare surveyed the company on their experience with the indicators. The survey reported the blood bag temperature indicators helped them significantly improve blood transportation quality control and workflow efficiency.
The Blood Temp 10 indicators helped Tenet Healthcare comply with regulatory guidance by having auditable proof blood core temperature was maintained when being returned to the blood bank.
Read the full case study below:
Tenet Healthcare Blood Transportation Case Study
Case Study verified by TechValidate.
Riverside Health System is a healthcare non profit that is using Blood Temp 10 temperature indicators for blood temperature compliance. PDC Healthcare surveyed the organization on the efficacy of the indicators. Their survey reported the blood bag temperature indicators helped them significantly improve blood transportation quality control and workflow efficiency.
In turn, the real time temperature monitoring increased patient safety and quality of care was improved.
Read the full case study below:
Riverside Health System Blood Temperature Compliance Case Study
Customer Evidence verified by TechValidate.
For more information on PDC Healthcare click here.
For the full survey on TechValidate click here.
PDC Healthcare surveyed Decatur Memorial Hospital about the benefits of using Blood Temp 10 indicators. Their survey found the blood bag temperature indicators used to measure blood core temperature helped the hospital improve the quality of care and significantly improve blood transportation quality control to comply with the FDA regulatory guidelines.
Read the full case study below:
Decatur Memorial Hospital Blood transportation Case Study
Customer References verified by TechValidate.
For more information on PDC Healthcare click here.
For the full survey on TechValidate click here.
Time and Temperature Indicator maker Timestrip launches the Timestrip® Temperature app, a new tool that provides a visual audit trail of its temperature products.
Frame
Zoom
Focus
Snap
Using the App simply involves taking a photo of a Timestrip® label with a smartphone.
If the Timestrip® has a new style serial number, the App will recognise the serial number and allow you to register it on the system.
You can then take a follow on scan where you can enter its activation state (inert, activated or breached), storing this data with essential time and location information in the cloud.
If the Timestrip® has an old style serial number you can still use the app in the same way but you'll need to manually enter the serial number. The App is free to download from the Google Play store with an iOS version to follow shortly.
High tech low cost
The App is the latest innovation from Timestrip, which revolutionised the field of cold chain management with its unique Irreversible Time and Temperature Indicator labels (TTIs).
Recognised by the World Health Organisation and the US FDA as an essential tool for the cold chain management of blood and food products, TTIs provide data as to whether a blood or food product is safe to use. Timestrip’s irreversible TTIs make this crucial safety information unambiguous and instantly visible.
Like having a hundred permanent digital data loggers
The main benefit of Timestrip’s irreversible TTI labels is that they give essential cold chain management information on individual units of processed products. This deals with the main shortcoming of digital data loggers, which are mostly used for containers of transported and stored products.
Thanks to the new Timestrip® App, extra key information around cold chain management is added with the safe storage of status, time, journey and location data, which can be accessed at any time via the app or via a website. A digital data logger can be lost or damaged and all its data can be wiped clean. The Timestrip® App however safely stores this data in the Cloud.
Data logger functionality at a fraction of the price
Timestrip UK CEO Reuben Isbitsky says: "I am very excited about our first App, which brings Timestrip TTI labels very close to data logger functionality, at a fraction of the price. While a key benefit of Timestrip products is that they do not need external infrastructure to read and interpret, using the optional Timestrip Temperature App to scan them, adds enhanced functionality, easy audit trail and provides the customer with a permanent record of the history of the product’s journey."
Interview with Reuben Isbitsky, founder of Timestrip UK Limited, which has just received FDA clearance for its 10°C Non-reversible Blood Transit Temperature Indicator.
Beyond its primary use as a temperature monitoring device, what from the user’s point of view are the key advantages for users of Timestrip 10°C nonreversible temperature indicators for blood products over other temperature monitoring products?
First and foremost, ease of use. Unlike other temperature monitoring devices, Timestrip 10°C irreversible blood temperature indicator can be stored at ambient temperature. It doesn’t need to be stored in a refrigerator and also doesn’t need to be refrigerated for at least 24 hours prior to use.
Moreover, until the button on the Timestrip 10°C is pressed, the device remains totally inactive. There is no risk of accidental activation because of the heat generated by warm fingers. All parts of Timestrip 10°C can be handled by warm hands without this leading to accidental activation. This naturally translates into significant cost savings over extremely sensitive temperature monitoring devices that need to be discarded when accidentally activated.
Ease to use temperature indicators
With Timestrip 10°C, there’s no complicated four-minute instructional video with many caveats. All you need to do is take the blood bag out of the cooler, put it down, take the Timestrip, peel off its protective under-layer, press the activation button and stick it on the blood bag. That’s it.
We’ve also built into Timestrip 10°C a fail-safe activation indicator that tells the user that the Timestrip is ready to use and working. This is unique in the market. No other indicator has this feature.
Lastly, we put huge emphasis on ease of readability. With Timestrip 10°C, if there’s a temperature breach, the indicator will turn from white to blue. Thus, if the indicator on the Timestrip has any hints of blue in it, the user knows for a fact that there’s been a temperature breach.
We don’t want there to be any confusion caused by problems of refracted colours from misleadingly coloured components of temperature monitoring devices indicating that a temperature breach has occurred, when in fact none has. This only leads to uncertainty, which at best means more costs to users because of unnecessarily discarded, but otherwise properly functioning monitoring equipment, and at worst viable blood is unnecessarily discarded.
Blood is an extremely precious commodity and no one wants to be using an unreliable temperature monitoring device that leads to throwing away good blood. As one of our users in Europe says: “Blood is life.”
Interview with Reuben Isbitsky, founder of Timestrip UK Limited, which has just received FDA clearance for its 10°C Nonreversible Blood Transit Temperature Indicator.
What does FDA clearance of Timestrip 10°C mean for your company?
It’s major milestone. Product-wise, we've been deemed and certified as having a medical device that has an accuracy level of ± 0.5°C and is suitable for transporting whole blood. FDA clearance for Timestrip 10°C will allow us to market this product for this specific application.
And for our business, it marks a very important turning point. We started off as a time indicator company, which evolved into a temperature monitoring business, that is now a high-end medical device and healthcare business.
Moving forward, our business model will be to configure new products for specific and invariably speciality uses. We’re also going to be expanding our range of products for specific uses to enable the safe transportation of the different components of whole blood as well, namely platelets, plasma and frozen blood products. This will go a long way to maximizing the usability of all these components. For instance, even properly transported and stored whole blood only has a shelf life of 35 days, but plasma can be frozen and has many uses.
Lastly, for markets outside the US FDA clearance gives our product enormous credibility. Although FDA clearance cannot be enforced outside the US, in fact for many markets such as Saudi Arabia and India, it is used as an indicator of credibility and for tendering processes is even a prerequisite.
Timestrip is proud to announce the successful certification to ISO 13485:2003 Quality Management Standard for Medical Devices in addition to our recent recertification of ISO 9001:2008.
Recognised around the world, the ISO 13485:2003 registration is based on eight quality management principles. These include: customer focus, leadership, involvement of people, process approach, system approach to management, continual improvement, fact-based decision-making and mutually beneficial supplier relationships.
The certification of the Company’s compliance with ISO 13485:2003 recognizes the policies, practices and procedures of our firm to ensure consistent quality in the products provided to our customers. With this certification, our medical and non-medical clients can be confident that Timestrip is dedicated to maintaining the highest efficiency and responsiveness achieving our ultimate goal – Guaranteed Customer Satisfaction.
Timestrip is certified as meeting the requirements of ISO:13485 for the following activities: DEVELOPMENT AND MANUFACTURING OF SMART LABELS FOR MEDICAL USE.
To maintain our certification, Timestrip will perform annual audits to ensure compliance and to assess initiatives for continued improvement. Our customers can be confident that Timestrip will continue to provide the high quality services they have come to expect well into the future.
We believe that our decision to become ISO 13485 Certified is a proactive one that not only anticipates the demands of our customers, but also demonstrates our commitment to providing quality services to all our customers in the medical industry. We strongly believe augmenting our certification offers our customers a “Best-in-Class” choice for smart labels.
The accreditation also supports our work in pharmaceutical categories, where a range of Timestrip products are used to identify temperature breaches in highly regulated cold chain environments. We are helping to protect patients and prevent unnecessary wastage of many drugs, vaccines and blood products, where the 2-8°C/46°F storage range is critical to so many products.
Timestrip indicators are leading the market in the provision of low-cost temperature indicators, as demonstrated by our blood bag temperature monitoring labels. In 2013 we launched the Timestrip Blood Temp 10 indicator, in response to the need to reduce wastage of precious blood stocks, discarded through uncertainty around temperature breaches incurred when bags are in use and removed from carefully controlled refrigeration storage units in blood banks.
In 2014, Timestrip UK Ltd announced it is working with Belgian Red Cross-Flanders to develop new blood temperature monitoring labels with a product development program looking to create optimal temperature monitoring labels for the blood collection, distribution and transfusion organisations operating within the Flanders catchment area.
Temperature monitoring labels based on the existing Timestrip Blood Temp 10 product are now being developed and reviewed in consultation with Belgian Red Cross-Flanders.
Also in 2014 we were proud to be the technology of choice for an innovative new product created to provide a wearable, low-cost vaccination reminder product for new-born children.
The Bill Gates Foundation funded Vaccine Reminder Band is currently undergoing field trials in Pakistan. Timestrip has a long history of innovation in the medical device sector and we now provide a range of products which act as invaluable visual aids to doctors, patients and healthcare workers.
For example in the UK, a number of NHS dispensing pharmacies are already using Timestrip® PLUS to monitor the cold chain for temperature-sensitive drugs such as Neulasta® and Total Paternal Nutrition (‘TPN’) products.
Timestrip is committed to quality assurance processes and procedures and we see the ISO 13485:2003 Quality Management Standard for Medical Devices accreditation as a natural progression of our work in the specialist markets in which we aim to excel.
Timestrip was on display at the Exhibit Hall in the impressive Pennsylvania Convention Center during the 2014 AABB Annual Meeting in Philadelphia, PA from the 25-28 of October.
Among the top medical devices and compliance tools for blood banks and hospitals being shown to AABB members from around the globe, our Blood Temp 10 product had its own display at the GenesisBPS booth.
We had fun meeting AABB members and demonstrating how our Blood Temp 10 product works.
Blood temperature breach indicator
Timestrip Blood Temp 10 is an irreversible, single-use 10ºC temperature breach indicator. It can be stored and shipped at room temperature when inert and is activated with simple finger pressure.
Once activated a confirmation window tells you the product is ready to monitor by filling with colour. It is then immediately adhered on the lower third of a pre-chilled blood bag.
Blood Temp 10 is ideal for the transportation of blood bags. When the core temperature of the blood breaches 10ºC, the indication window turns blue, showing you the blood is no longer suitable to return to storage.
If you missed out seeing us at the AABB 2014 Annual Meeting, you can read more about Blood Temp 10 here.
If you liked the clear breach indication and ease of use of Blood Temp 10, but need to monitor extreme room temperature breaches, then have a look at our Timestrip Plus ascending temperature breach indicators here.