Time and distance are the twin challenges when managing cold chain logistics for the transportation of fresh food perishables
Fruit and vegetables, packaged salads, dairy produce, eggs and even flowers – consumers the world over depend on supply chains that can bring perishable goods to retailers’ shelves speedily and in the best possible condition. Their instantly made purchasing decisions depend on products looking as appealing and unblemished as they do in promotional advertising.
At Timestrip, we see this every day. One of our aquaculture clients uses our Irreversible Time and Temperature Indicators in the export of seafood to the US market. He reports that how his products look upon arrival directly impacts on whether a delivery is rejected or not. His use of Timestrip smart labels ensures that his cold chain management processes guarantee not only that his products are safe after transport, but also look fresh to the end-user. (Read our Ferme Marine de Mahebourg case study)
Moreover, this need for efficient transport processes that guarantee product quality at the point of sale is playing itself out against a backdrop of rising worldwide consumer demand for perishable goods as per capita incomes rise in major markets such as China and India.
A general rule of thumb in the global food trade is that lower per capita income nations tend to import mostly grains, while higher per capita income nations import a wide range of cold chain perishables. So, while 70% of foods consumed in the US are managed by cold chain processes against only 25% of the meat and 5% of the fruits and vegetables in China, for the latter these figures are rising rapidly.
Also, within developed markets, the demand for perishables is rising. According to Winnesota Regional transportation, the US refrigerated transportation market on which the sale of perishables depend is growing at a rate of around 12.5% per annum, with strong projected growth in the coming years.
Projected annual growth in global cold chain to 2020 - 13.9%
Fresh produce shelf-life time spent in transit- 50%
Source: Logistics Bureau
Although regulation around safety is an important factor in the growth of cold chain transportation, globalisation and innovation play a far more important role. Technological progress around refrigerated transport makes it possible for less developed economies to sell their perishable produce in far away markets.
Complex international supply chains
As soon as a flower is picked or a fruit and vegetable is harvested, it begins to rot. Time and distance are thus the twin challenges of the fresh cold chain.
In fact for some produce such as Guatemalan bananas that picked when they are green, this biological process occurs during transit thus ensuring they arrive at retailers in the EU and the US in a saleable condition.
To avoid product spoilage, speed is the number 1 priority. Cold chain processes are therefore equipment and technology intensive in order to prolong freshness so consumers get to buy saleable produce.
Other considerations include:
- product sensitivity to weather and other naturally occurring disruptions
- the maintenance of sound sanitary practices from producer to retailer
- the requirement of specialised handling and packaging
- accurate labeling and traceability
Faster supply chains
An increasingly common feature of making perishables supply chains faster is for major supermarkets to take total control of this process with acquisitions of farming production facilities and the development of close business relationships with growers.
In the UK, the supermarket chain Morrisons is also one of the country’s biggest food manufacturers, with over 20 nationwide food production and distribution sites. The group says that more direct links with its farmers enable it to deliver the freshest food and best value to its customers.
Vertical supply chains also allow for growers and farmers to earn more from their produce. This happens in France with the supermarket chain Intermarché whose close relationships with dairy farmers ensure that they are paid 50% of the retail sales price of a litre of branded milk.
From farm to supermarket shelf
The Leckford Estate in Hampshire, southern England, is the Waitrose supermarkets farm that supplies its produce to Waitrose stores across the UK
Keeping the fresh cold cold chain cool
Although speed is essential in ensuring that the transport and delivery of perishables avoids spoilage, temperature control is another vital component in guaranteeing fresh cold chains.
“Maintaining the cold chain is one of the most effective ways to ensure safe, quality food”
Jorge Hernandez, Senior VP, food safety and quality assurance, U.S. Foods
The complexity of the required processes has led to the food companies working increasingly with specialist third party suppliers and the requirement of suitably qualified personnel.
Another key cold chain area for temperature monitoring revolves around equally specialist technologies, such as RFID tags that record and forward real-time temperature data, real-time GPS tracking that provide visibility of shipment progress, and a range of high-tech time and temperature monitoring solutions.
Avoiding waste due to spoilage
Fresh produce spoils easily and in many cases this is only identified at the very end of the transportation cycle, leading to either shipments being rejected or consumers claiming refunds from retailers. Moreover, apart from waste caused by the spoilt produce, further costs are incurred related to transporting these products that will never be consumed.
For companies, these have a big impact on their financial results. Timestrip Irreversible Time and Temperature Indicators are currently being used by a number of companies to address this issue. In the US for instance, the rail carrier Amtrak has integrated Timestrip products to successfully reduce food waste of its on-board catering services. Read our Amtrak case study.
At an industry level, a new vision about how to reduce these costs is around the establishment of "lean practices" whereby handling by various stakeholders in a perishables supply chain are kept to the very minimum. This is part of the thinking behind supermarkets taking control of food production mentioned above.
A strategy in this area includes a focus on direct-stores deliveries that avoid traditional centralised distribution methods and the maintenance of smaller local fresh produce warehousing much closer to retail outlets.
Significant savings can be also generated with appropriate packaging that protect fragile perishables against damage and innovative materials that actually extend the life of produce by controlling its life-cycle with the use of ripening agents such as ethylene gas.
This can involve speeding up the ripening process with innovative packaging containing ethylene gas capsules that can ripen fruit on demand, and ethylene gas that is used to ripen green bananas during transportation. It can also involve slowing down the ripening process with the use of ethylene absorbers that are added to fruit packaging.
A growing area of cost savings in fresh supply chains is the use of returnable plastic crates (RPCs) that can be used many times.Their ergonomic design allows for space-saving stacking, safer handling and ventilation and draining hat reduces in-transit spoilage. Plus with so-called "one-touch merchandising", RPCs placed directly onto shelf displays,further reducing handling.
They also have an impressive environmental record: a 2013 study found that RPCs generate 82% less waste, consume 92% less water, cause 76% less ozone depletion and require 49% less energy.
Managing the fresh cold chain
Franck Artis is the Singapore Branch Manager of Food Distribution Pte Ltd. He says:
For perishables such as dairy produce, it is essential that the cold chain is not breached during transportation, with an ideal temperature range of 3°C to 4°C. This is just as much for product quality as for food safety. Normally this must involve some kind of refrigerated transport.
For domestic transport such in France, this involves refrigerated lorry deliveries direct to retail outlets or (often regional) distribution platforms that have dedicated cold rooms for temporary storage. For export, this can involve either a transporter operating door-to-door processes or alternatively outsourcing this function to third-party cold chain logistics suppliers.
A key feature of cold chain management during the maritime transport of perishables is the ability of transporters to supply accurate and complete temperature data that shows that the cold chain has not been breached. This can mean using reefer containers with temperature tracker boxes that monitor and control the temperature inside the containers, and log and respond to any breaches, say, in the event of a temporary power outage.
The economics of the transport of perishables dictate that temperature monitoring tools such as Irreversible Time & Temperature Indicators would only be used for high added value items, such as caviar, rather than a carton of yogurt or milk.
For perishables such as flowers, avocados, bananas and tomatoes, transit time is used a ripening period to ensure that when they arrive at their final destination (retail) point, they are fit for sale.
The advent of the banana cold chain
Bananas make up the largest share in the transport of perishables, equivalent to approximate 20% in volume and worth some US$8bn in 2013.
Exact figures of global banana production are difficult to determine as globally only 15% of production (which include plantain) is exported and much of what is not exported is produced by small or very small growers.
Nevertheless, refrigerated cargo (aka reefer) ship technology has been an important driver in the cold chain technology development. The first reefer ship for the transport of bananas was developed in the US in 1902 by United Food Company.
Up until that point, bananas were a highly exotic fruit and very much a luxury item in non-banana producing nations.
Today, it is one of the world's most consumed fruit and due to its distinct ripening process, the trade and transportation of this produce has led to distinct term in cold chain logistics – the so-called 'banana cold chain'.
Whether it’s Chilean blueberries for breakfasts in London, French-made polio vaccines for use in Malawi, or Mauritian sea bass for restaurants in the Big Apple, the chances are that all these cold chain perishables have been transported by air.
Indeed, thanks to our increasingly connected world and rising incomes in developing nations, consumers across the globe are opting for perishables produced far from where they will be consumed. Since 2010 in India for instance, rising per capita incomes have led to an increase in the consumption of frozen food, meat, fish, canned and instant food items, as well as a greater acceptance of frozen vegetables.
Similar trends have been noted in China, where in increasing numbers more affluent consumers are opting for imported foodstuffs, especially seafood.
Strong growth for cold chain logistics
For cold chain pharma products, the figures are startling. In its annual Biopharma Cold Chain Sourcebook, Pharmaceutical Commerce estimates the global volume of 2017 cold chain products at $283 billion, out of a total market of $1.17 trillion, and growing at approximately twice the rate of the overall pharma market.
The International Air Transport Association (IATA), which supports aviation with global standards for airline safety, security, efficiency and sustainability, estimates that immunization prevents 2.5 million deaths every year and sees air cargo as critical for flying short shelf-life vaccines to their destination in time to be effective.
Air transportation is also critical to the economy of many regions, notably fruit‐ and vegetable‐producing countries in Africa that ship most of their produce to developed markets.
Time and Temperature Indicators (TTIs) play a key role in this process, by providing clear and unambiguous data as to whether any cold chain breaches have occurred during transit, and if so, how long they lasted. Moreover their use with certain products in certain markets are mandated by regulatory authorities, such as by the FDA for seafood imports into the US.
Managing complex logistics
That said, the transportation of cold chain perishables by air is highly complex and prone to numerous situations where temperature breaches can occur. In a study for the air transporter IAG Cargo, researchers found that over half of all temperature deviations occurred during transportation.
This data is supported by findings of the World Health Organisation (WHO), which says: “The greatest and most frequent vulnerability to temperature exposure occurs on the airport tarmac when goods are exposed to the elements before aircraft loading, or during unloading.”
Research however points to cold chain logistics managing these situations effectively for some time now. A 2012 study of Icelandic fish exported to the UK and France showed that despite poor temperature control during storage and ground operations at the airports before and after the flights, relatively a moderate increase in the temperature of the fish were recorded: the pallets of food were subjected to ambient temperatures above 10°C as well as solar radiation for several hours, but the temperature of the fish rose by less than 2°C. This was explained by the transportation of the fish inside polystyrene boxes, which were kept cool with ice inside the boxes.
It’s important to stress though that in these sorts of situations it’s only the use of temperature monitoring tools such as irreversible TTIs inside the boxes on the transported items themselves that a stakeholder can know whether potentially damaging temperature breaches have occurred.
Food loss epidemic
Nevertheless, despite much progress and professionalisation in cold chain logistics, certain problems remain difficult to resolve. One of the main costs with the transportation of perishable products such as fruit and vegetables is wastage due to spoilage related to inadequate temperature management during transit. Estimates vary as to what this amounts to, but most data suggests it is in the region of 33%.
According to the United Nations’ Food and Agriculture Organization (FAO), this wastage should be viewed as a “food loss epidemic”, which it values at about $1 trillion each year. Added to this are related environmental costs: wasted water used to produce food that is never eaten equal to the water needs of Africa, and CO2 emissions equivalent to removing every car off the road across the world.
It is hard to imagine any industry would tolerate 30-40% inefficiency.
Food bacteria grow best between 5°C and 60°C, which explains why keeping perishable products cool, cold, frozen or deep frozen is the only way to guarantee product quality and shelf-life as it arrives at the end of a transportation process.
Customers of air transport providers for perishables are well aware of this. In a survey of their various concerns, the three greatest were recorded as:
- the expertise of handling personnel
- appropriate temperature monitoring
- traceability
What is remarkable about these concerns is how they very much match those of the healthcare sector and it would seem that moving forward, it will be the lessons learnt from transporting pharma products, that could help avoid the massive cost of food spoilage during transit.
In a conference in Dallas, Texas, last year organised by the Netherlands-based Cool Chain Association, chairman Stavros Evangelakakis suggested applying healthcare industry standards to perishables would lead to wastage being “dramatically lowered”.
A post conference statement went on to stress the need to “treat perishable cargo with the same care, respect and transparency as pharma”, adding that this would be “crucial” for new and emerging markets such as South American and Africa.
Potential “quadruple win” for cold chain logistics
A recent study reported on in The Guardian highlights the huge benefits proper cold chain management can bring to the transport of perishable foods.
A carrier in India field tested cold chain equipment with a local grower for the transport of fruits in refrigerated trucks from Punjab to Bangalore, a 1,600 mile-trip over rough roads in high temperatures.
The results were significant:
- a one-week shelf life increased to two months
- profit increases by up to 23% for all the supply chain actors
- post-harvest food loss reduced by 76%
- greenhouse gas emissions reduced by 16%, excluding the significant reduction of emissions from food loss
Blueberries in January
One of the massive economic benefits of effective cold chain management is how it enables the creation of new markets.
In most countries, blueberries used to be only available at specific times of the year. The situation changed dramatically in the 1980s, with Chile harvesting blueberries from October until late March and exporting these to the US.
Today thanks to climate-controlled storage and transport technologies, the fruit is available year-round in many regions, buoyed on by the rebranding of the fruit as a “super-food”.
Today, the US is the world’s largest producer of blueberries, followed by Canada, Poland and France. Meanwhile in the UK, 2010 was the year blueberries overtook raspberries as the country’s favourite soft fruit after strawberries.
What is Timestrip Complete?
Timestrip Complete is our product that monitors upper and lower threshold temperature breaches, such as 2°-8°C cold chain monitoring for vaccine storage and transport.
The product is in fact made up of two elements: a Timestrip Plus, which is our ascending indicator that measures, for example temperature breaches above 8°C and the amount of time that they have breached, and a descending temperature indicator called Freeze Check Plus FCP for short that logs breaches, for example, below 2°C.
This is why we’ve called the indicator “Timestrip Complete”: It provides complete monitoring for the 2°-8°C cold chain.
Why was Timestrip Complete developed for vaccine storage and transport?
A vaccine is affected by heat and by freezing and the two critical temperature thresholds for storage and transport are 2°C and 8°C.
It’s time and temperature that’s the critical factor leading to degradation of vaccines.
For freezing, the crucial event is whether this has happened or not. Because vaccines are contained in an aqueous solution (water), when it freezes, it expands and the resulting crystallization can destroy the vaccine
How much does a Timestrip Complete cost?
Under US $4 per unit, and considerably less in volume purchase. It’s the lowest cost solution and easiest to implement to achieve complete 2°C-8°C temperature monitoring. Any other tool that does this job involves using a single-use digital data logger, which start at about US$10 per unit
This totally fits in with our company ethos of providing the easiest, simplest and most cost-effective products for temperature monitoring. Many 0°C or 2°C descending indicator on their own for instance normally costs at least US$2.50, so we know that our sub-US $4 price is very competitive.
How do you use Timestrip Complete?
It can be stored at room temperature, is inert until activated and has a button-like feature that needs to be pushed for activation.
There is an extra measure needed in storage to bear in mind— and this is industry-wide for all descending temperature indicators — is that they are always active.
This means they need to ship to end-users above the temperature they monitor and we achieve this by packing them with heat packs to ensure they never go below 2°C.
On the positive side from our clients point of view, because the ascending indicator can be stored at room temperature, they only need to ensure that our products are kept above freezing.
Similar products present the logistic difficulties of needing to be kept above 2°C and below 8°C, so this is a big advantage
Are there other Timestrip Complete products apart from 2°C-8°C?
Yes, this is a key range which we are looking to grow. The product also comes as 0°C-8°C
Timestrip Complete 0°C - 8°C and 2°C - 8°C are also used for the storage and transportation of medicine and biologics.
We are now launching a Controlled Room Temperature indicator that has 15°C and 25°C thresholds. These are designed for environments such as food transportation for airlines.
The key message here though is that we have the technology to produce a variety of complete upper and lower level temperature monitoring — and at a very competitive price.
What’s been the feedback so far from end-users of these complete temperature monitoring indicators?
Timestrip was involved in supporting anti-rabies work in Malawi with a 0°C-30°C indicator for the transportation and storage of canine vaccine. Rabies is huge problem in Malawi and spoilt vaccines seriously hamper efforts to address the situation.
The bottom line was that this 0°C-30°C product was successful in identifying those doses of canine vaccine that were either spoilt because of ambient heat exposure or from freezing caused by the ice-packs that are used during transportation.
This allowed for a re-stocking with unspoilt vaccines that ensured that the anti-rabies field teams’ efforts were effective and thereby measurably supported the prevention of more cases of rabies in Malawi.
Abstract: The World Health Organisation stresses the importance of maintaining the cold chain for temperature sensitive vaccines. Due to the recommendation for rabies vaccines to be stored between 2° and 8° Celsius and the nature of canine mass vaccination projects, cold chain maintenance remains one of the most significant logistical hurdles project managers have to overcome.
Mission Rabies field teams in Malawi evaluated the use of adhesive temperature monitors as an alternative to thermometers in both storage fridges and field cooler boxes during the duration of a 4 week mass vaccination drive in April/May 2016.
Two different Timestrip® temperature monitors were used to alert the teams to the critical temperature thresholds of 0°C and 30°C. As freezing rapidly damages vaccines, each vaccine box containing 10 vials of 10ml vaccine was marked with a monitor that indicates the immediate crossing of the lower threshold through colour change. As damage from high temperatures is related to both the temperature achieved and the time of exposure, to test for the upper temperature limit each cooler box was equipped with a monitor that showed the crossing of the upper threshold over time.
Of a total of 22 vaccination teams participating in the study, 7 teams reported an exposure of the vaccines to temperatures above 30°C for more than 4 hours and on 9 occasions teams reported a temperature drop to 0°C or below.
These findings highlight the importance of monitoring the temperature during field campaigns, both due to proximity to ice packs and exposure to high outside temperatures. The use of Timestrip temperature indicators gives early visual clues and raises awareness among the team. However practicability of the temperature monitoring must be taken into consideration and adhesive monitors need to be improved with regards to their setup to make them a valuable tool in field projects.
This abstract was presented at the 28th Annual Rabies in The Americas Conference on 23 October 2017.
Authors: Frederic Lohr, Reuben Isbitsky, Andy Gibson, Alasdair King
Timestrip will be attending the International Association for Food Protection – IAFP - in Tampa, Florida from 9th – 11th July 2017.
We will be demonstrating our 3°C Seafood, 5°C, 8°C & 10°C irreversible ascending temperature indicators at the exhibition. We will be at booth 847, please come and get a sample of our Nonreversible Food Temperature Indicators to test and take away with you. Click here for more information.
Food temperature monitors
Timestrip temperature monitoring labels make tracking temperature breaches across a multitude of cold chain food industry applications a simple, cost-effective process.
In fact, we offer the most cost-effective solution of our type in food standard and food safety monitoring, which is why our technology has already been adopted by a number of innovative businesses. Our precise, efficient, user-friendly temperature indicator labels are helping to ensure effective cold chain management around the world.
Each year, the International Association for Food Protection hosts an Annual Meeting, providing attendees with information on current and emerging food safety issues, the latest science, innovative solutions to new and recurring problems, and the opportunity to network with thousands of food safety professionals from around the globe. Held in various locations throughout North America, this meeting has grown over the years to become the leading food safety conference worldwide.
Timestrip UK Ltd have expanded their range to include their latest Timestrip PLUS™ 10°C threshold indicator with a 7-day run-out/recording window. This specific indicator is meeting the demand within the pharmaceutical industry and the regulations and compliance that come with transportation and storage. With Timestrip’s latest 10° 7 day offering, they are reaching out further within the Pharmaceutical and Healthcare industry and ensuring their high tech but low cost devices are evolving with the relevant advances in the industries.
7 Day Pharmaceutical temperature breach indicator
Timestrip design and manufacture innovative, irreversible time and temperature indicators for a range of industries from Food to Pharmaceutical. Their Timestrip® PLUS range is predominantly used across the Healthcare, Life sciences and Pharmaceutical arena. Currently, the Timestrip PLUS™ range covers temperature thresholds from -20°C up to 38°C and generally display a run-out window of any temperature breach for 8, 12 or 48 hours.
Combining time & temperature monitoring patented technology, which involves controlled rate lateral diffusion of liquids through the device membrane once activated. If a breach occurs, the ‘smart’ indicators will visually indicate how long a product has been exposed to higher temperatures.
For a free sample of the new 10° 7 day indicator, or to place an order click here
Visit the Timestrip Food Indicator page for solutions
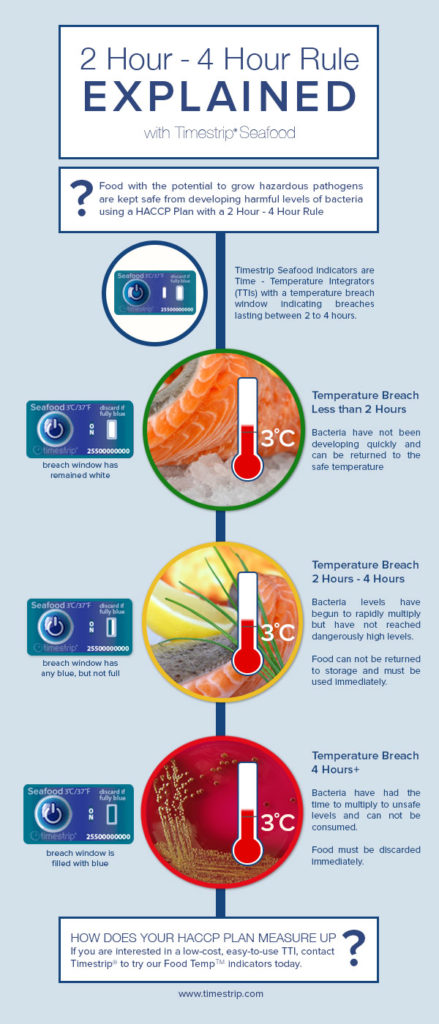
Maintaining a correct cold chain is essential in managing the supply of seafood products in order to avoid contamination and food poisoning, especially from C Botulism. For restaurants and retailers, who are towards the end of a typical cold chain, ensuring this has occurred prior to delivery is a particular concern. Failure in this area can have dire consequences, both in cost and PR terms, and for consumers, they can be extremely dangerous and in some cases lethal.
C Bot seafood recall alert
In June for instance, the FDA website published an alert about a Florida-based supplier of tuna products that was obliged to initiate a US-wide product recall due to suspected C Botulism contamination of its product.
The manager of one major London restaurant says that the main ways his establishment monitors the quality of the seafood it orders are visual and olfactory inspection of the product upon receipt and the use of trusted suppliers that have the correct certification. The products are also checked upon receipt with temperature probes.
He says that a contaminated seafood product will “not look right”, that it will look “sweaty” and for fish as opposed to shellfish, that the eyes of fish that have “gone off” will look “dead looking”.
He also says that contaminated fish will have a distinct unpleasant smell and that his rule of thumb is “discard fish that smells fishy”.
He says that the scenario of the refrigerated delivery truck whose engine was switched off for a number of hours prior to delivery needn’t necessarily lead to a fall in the refrigerated ambient temperature within it as long as the truck’s doors haven’t been opened.
He adds that major points of sale of seafood products such as Billingsgate market in south-east London have power points specifically to ensure that the refrigeration units of delivery trucks are kept on, thus ensuring that the seafood products they are carrying are kept at the correct cooled or frozen temperatures.
He does concede though, that for oysters specifically, in his 40 years’ experience as a restaurant professional, statistically all restaurants that offer them will occasionally serve oysters that lead to food poisoning.
Lastly, he says that in his experience restaurants are extremely rigorous in the correct application of good food management principles and most cases of food poisoning from contaminated seafood in fact occur in private residences where there is much less awareness of these principles.
Furthermore, he says that among people who aren’t professionals of the food industry, there is also a poor understanding of food poisoning and in all suspected cases enquiries are always made about what was eaten in the previous 72 hours as invariably the cause of the incident was not what was last consumed.
Across the Channel in France, a similar approach also exists with a specialist Marseille-based seafood restaurant and retailer that has two busy branches and has been operating for over 60 years.
The manager of this business, who deals the most with quality and cold chain monitoring, says for him visual inspection of seafood products upon receipt of new stock is his most useful tool to determine whether an appropriate cold chain has been maintained.
He says fish that is spoiled due to not having been kept at the correct cooled temperature will “not feel right” and “not have the right firmness”. This, he says, occurs even before the product starts to smell bad.
He also knows that fish that is spoiled always have eyes that are not the correct colour, which is another indication that they haven't been kept at the correct chilled temperature prior to delivery.
Another aspect of how his restaurant ensures the quality of the products they serve is to only use trusted suppliers and not to deal with intermediaries. Because of these strong longstanding relationships, his business benefits from a “no questions asked” returns policy.
He also says experience is also key, pointing to the 60+ years his establishment has been in existence.
However, like his counterpart in the UK, beyond many years’ professional experience and the use of trusted suppliers and mainly visual inspection of seafood products upon receipt, no cold chain monitoring tools are used.
Both also were emphatic about stressing the value of their respective establishment’s reputation and how damaging an outbreak of food poisoning would be. This, they both said, was always one of the main issues at the front of their minds when they managed their seafood products.
However at one of Marseille’s main teaching hospitals, one senior nurse was extremely dubious about how restaurants manage and monitor seafood cold chains. Her many years’ clinical experience was that they often cut corners and few would discard products that might be spoiled, but could not be returned, due to the appropriate cold chain not having been maintained. She also said she thought this extremely worrying situation would occur even if sophisticated temperature indicators were used at all stages of the seafood cold chain.
Timestrip is proud to announce the successful certification to ISO 13485:2003 Quality Management Standard for Medical Devices in addition to our recent recertification of ISO 9001:2008.
Recognised around the world, the ISO 13485:2003 registration is based on eight quality management principles. These include: customer focus, leadership, involvement of people, process approach, system approach to management, continual improvement, fact-based decision-making and mutually beneficial supplier relationships.
The certification of the Company’s compliance with ISO 13485:2003 recognizes the policies, practices and procedures of our firm to ensure consistent quality in the products provided to our customers. With this certification, our medical and non-medical clients can be confident that Timestrip is dedicated to maintaining the highest efficiency and responsiveness achieving our ultimate goal – Guaranteed Customer Satisfaction.
Timestrip is certified as meeting the requirements of ISO:13485 for the following activities: DEVELOPMENT AND MANUFACTURING OF SMART LABELS FOR MEDICAL USE.
To maintain our certification, Timestrip will perform annual audits to ensure compliance and to assess initiatives for continued improvement. Our customers can be confident that Timestrip will continue to provide the high quality services they have come to expect well into the future.
We believe that our decision to become ISO 13485 Certified is a proactive one that not only anticipates the demands of our customers, but also demonstrates our commitment to providing quality services to all our customers in the medical industry. We strongly believe augmenting our certification offers our customers a “Best-in-Class” choice for smart labels.
The accreditation also supports our work in pharmaceutical categories, where a range of Timestrip products are used to identify temperature breaches in highly regulated cold chain environments. We are helping to protect patients and prevent unnecessary wastage of many drugs, vaccines and blood products, where the 2-8°C/46°F storage range is critical to so many products.
Timestrip indicators are leading the market in the provision of low-cost temperature indicators, as demonstrated by our blood bag temperature monitoring labels. In 2013 we launched the Timestrip Blood Temp 10 indicator, in response to the need to reduce wastage of precious blood stocks, discarded through uncertainty around temperature breaches incurred when bags are in use and removed from carefully controlled refrigeration storage units in blood banks.
In 2014, Timestrip UK Ltd announced it is working with Belgian Red Cross-Flanders to develop new blood temperature monitoring labels with a product development program looking to create optimal temperature monitoring labels for the blood collection, distribution and transfusion organisations operating within the Flanders catchment area.
Temperature monitoring labels based on the existing Timestrip Blood Temp 10 product are now being developed and reviewed in consultation with Belgian Red Cross-Flanders.
Also in 2014 we were proud to be the technology of choice for an innovative new product created to provide a wearable, low-cost vaccination reminder product for new-born children.
The Bill Gates Foundation funded Vaccine Reminder Band is currently undergoing field trials in Pakistan. Timestrip has a long history of innovation in the medical device sector and we now provide a range of products which act as invaluable visual aids to doctors, patients and healthcare workers.
For example in the UK, a number of NHS dispensing pharmacies are already using Timestrip® PLUS to monitor the cold chain for temperature-sensitive drugs such as Neulasta® and Total Paternal Nutrition (‘TPN’) products.
Timestrip is committed to quality assurance processes and procedures and we see the ISO 13485:2003 Quality Management Standard for Medical Devices accreditation as a natural progression of our work in the specialist markets in which we aim to excel.
Timestrip was on display at the Exhibit Hall in the impressive Pennsylvania Convention Center during the 2014 AABB Annual Meeting in Philadelphia, PA from the 25-28 of October.
Among the top medical devices and compliance tools for blood banks and hospitals being shown to AABB members from around the globe, our Blood Temp 10 product had its own display at the GenesisBPS booth.
We had fun meeting AABB members and demonstrating how our Blood Temp 10 product works.
Blood temperature breach indicator
Timestrip Blood Temp 10 is an irreversible, single-use 10ºC temperature breach indicator. It can be stored and shipped at room temperature when inert and is activated with simple finger pressure.
Once activated a confirmation window tells you the product is ready to monitor by filling with colour. It is then immediately adhered on the lower third of a pre-chilled blood bag.
Blood Temp 10 is ideal for the transportation of blood bags. When the core temperature of the blood breaches 10ºC, the indication window turns blue, showing you the blood is no longer suitable to return to storage.
If you missed out seeing us at the AABB 2014 Annual Meeting, you can read more about Blood Temp 10 here.
If you liked the clear breach indication and ease of use of Blood Temp 10, but need to monitor extreme room temperature breaches, then have a look at our Timestrip Plus ascending temperature breach indicators here.