How Timestrip irreversible time sensitive indicators are used in Germany by Ulmer Wohnungs for their apartment ventilation units to remind tenants that they need to change the ventilation duct filters.
Translation: "In many UWS apartments, ventilation units are installed which permanently ensure fresh and clean air. Only when the filter is clean, do the fans work quietly and use less energy. In our
Timestrip Time Sensitive indicator labels
Timestrip can make you a custom Time Sensitive Indicator Label - we create custom solutions that measure time ranging from 1 hour up to 2 years. We are ISO 9001:2015 certified and Timestrip time indicators offer a helpful visual reminder of service and maintenance schedules for consumable components. Accurate and reliable, Timestrips contribute to a more positive experience for the consumer and help ensure optimum performance from appliances that need replacement indicators or service reminder indicators in order to function effectively.
Timestrip is proud to announce the successful certification to ISO 13485:2003 Quality Management Standard for Medical Devices in addition to our recent recertification of ISO 9001:2008.
Recognised around the world, the ISO 13485:2003 registration is based on eight quality management principles. These include: customer focus, leadership, involvement of people, process approach, system approach to management, continual improvement, fact-based decision-making and mutually beneficial supplier relationships.
The certification of the Company’s compliance with ISO 13485:2003 recognizes the policies, practices and procedures of our firm to ensure consistent quality in the products provided to our customers. With this certification, our medical and non-medical clients can be confident that Timestrip is dedicated to maintaining the highest efficiency and responsiveness achieving our ultimate goal – Guaranteed Customer Satisfaction.
Timestrip is certified as meeting the requirements of ISO:13485 for the following activities: DEVELOPMENT AND MANUFACTURING OF SMART LABELS FOR MEDICAL USE.
To maintain our certification, Timestrip will perform annual audits to ensure compliance and to assess initiatives for continued improvement. Our customers can be confident that Timestrip will continue to provide the high quality services they have come to expect well into the future.
We believe that our decision to become ISO 13485 Certified is a proactive one that not only anticipates the demands of our customers, but also demonstrates our commitment to providing quality services to all our customers in the medical industry. We strongly believe augmenting our certification offers our customers a “Best-in-Class” choice for smart labels.
The accreditation also supports our work in pharmaceutical categories, where a range of Timestrip products are used to identify temperature breaches in highly regulated cold chain environments. We are helping to protect patients and prevent unnecessary wastage of many drugs, vaccines and blood products, where the 2-8°C/46°F storage range is critical to so many products.
Timestrip indicators are leading the market in the provision of low-cost temperature indicators, as demonstrated by our blood bag temperature monitoring labels. In 2013 we launched the Timestrip Blood Temp 10 indicator, in response to the need to reduce wastage of precious blood stocks, discarded through uncertainty around temperature breaches incurred when bags are in use and removed from carefully controlled refrigeration storage units in blood banks.
In 2014, Timestrip UK Ltd announced it is working with Belgian Red Cross-Flanders to develop new blood temperature monitoring labels with a product development program looking to create optimal temperature monitoring labels for the blood collection, distribution and transfusion organisations operating within the Flanders catchment area.
Temperature monitoring labels based on the existing Timestrip Blood Temp 10 product are now being developed and reviewed in consultation with Belgian Red Cross-Flanders.
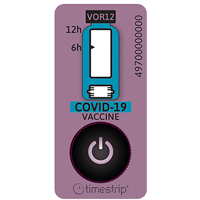
Also in 2014 we were proud to be the technology of choice for an innovative new product created to provide a wearable, low-cost vaccination reminder product for new-born children.
The Bill Gates Foundation funded Vaccine Reminder Band is currently undergoing field trials in Pakistan. Timestrip has a long history of innovation in the medical device sector and we now provide a range of products which act as invaluable visual aids to doctors, patients and healthcare workers.
For example in the UK, a number of NHS dispensing pharmacies are already using Timestrip® PLUS to monitor the cold chain for temperature-sensitive drugs such as Neulasta® and Total Paternal Nutrition (‘TPN’) products.
Timestrip is committed to quality assurance processes and procedures and we see the ISO 13485:2003 Quality Management Standard for Medical Devices accreditation as a natural progression of our work in the specialist markets in which we aim to excel.
Frostine's: Major Korean beauty brand LG's skin care line
Timestrip indicators were added to the base of glass bottles used across the Frostine’s range by LG cosmetics as it launched its revolutionary new brand across Korea in one of its biggest multi-channel campaigns of 2012.
LG is the second biggest cosmetic brand owner in Korea and the Frostine’s brand extends its reputation for innovation in the sector.
The Frostine's range comprises toners, eye creams, masks and moisturizers with a unique formulation that requires the products to be kept refrigerated at all times.
Skincare efficacy timer label
In addition to the chilled characteristics, some of the creams and toners lose their efficacy after 6 weeks, so each bottle comes with a Timestrip applied to the base so that consumers can trigger the timing label and check the product integrity at a glance each time they are used.
See their custom Timestrip design and unique cosmetic integration from 1:50-3:00 in the video.
These Timestrip time monitoring indicators, safe for use in refrigerated environments, are now incorporated into Vegellia beauty products.
Skincare lotion shelf life reminder
Users will see a visual indication of the elapsed time reminding them to replace their Vegellia lotion at the end of the product's shelf life. The Timestrip indicator ensures users will always be using Vegellia products at their top performance.
LG is a cosmetic company creating unique formulations with natural ingredients, passing on the lessons of nature in the way certain treatments work best at chilled temperatures.
Temperature skincare storage reminder
They want their consumers to experience their products in optimum condition and chose Timestrip to help communicate the time-sensitive nature of the Frostine’s Icemetic range.
See their custom Timestrip design and unique cosmetic integration from 1:50-3:00 in the video above.
Timestrip indicators have been added to the base of glass bottles used across the Frostine’s range by LG cosmetics as it launches the revolutionary new brand across Korea in one of its biggest multi-channel campaigns of 2012.
LG is the second biggest cosmetic brand owner in Korea and the Frostine’s brand extends its reputation for innovation in the sector.
Fridge storage timer for skincare
The Frostine's range comprises toners, eye creams, masks and moisturizers with a unique formulation that requires the products to be kept refrigerated at all times. In addition to the chilled characteristics, some of the creams and toners lose their efficacy after 6 weeks, so each bottle comes with a Timestrip applied to the base so that consumers can trigger the timing label and check the product integrity at a glance each time they are used.
The contract order comprised of 100,000 six-week indicators, with the Frostine’s branding overprinted onto each Timestrip.
The range is being retailed through selected stores and via the Frostine’s website, with consumers able to buy range collections shipped in their own mini-refrigerator. Products in the range start at around £30 each.
CVS has released a new toothbrush incorporating Timestrip® technology that informs the user when to replace their brush. Dentists recommend changing your toothbrush every 3 months.
Toothbrushes used after 3 months become worn and much less effective at removing plaque which can lead to dental health problems. Plaque can be reduced to up to 30% when toothbrushes are replaced ever 3 months.
Timestrip toothbrush replacement reminder
The CVS Timestrip Extra® model has a window that shows users when their brush is past its peak, as the window on the brush will fill until it hits the "replace" printed on the toothbrush handle. The Timestrip® replacement reminder is more-effective than the method which bristles change colour, because bristles can be affected by certain water types and toothpastes. (more…)
Tesco has released their revolutionary new Pro-Tech model toothbrush incorporating Timestrip® technology that informs the user when to replace their brush. Dentists recommend changing your toothbrush every 3 months. Toothbrushes used after 3 months become worn and much less effective at removing plaque which can lead to dental health problems.
Timestrip tells you when to replace
The Tesco Pro-tech model has a window that shows users when their brush is past its peak, as the word "replace" appears. This Timestrip® replacement reminder is more-effective than the method which bristles change colour, because bristles can be affected by certain water types and toothpastes.
(more…)